Diagnostic tests for Clostridioides difficile
Clostridioides difficile infection
Clostridioides difficile (C. difficile) is the major cause of nosocomial diarrhoea and pseudomembranous colitis. The bacterium produces spores and infection usually occurs when antibiotics alter or kill the normal gut microflora, allowing the germination of spores into vegetative cells. Once C. difficile growth begins, toxins A and B may be produced, causing diarrhoea and colitis. Rapid, accurate diagnosis of diarrhoea caused by C. difficile is central to a patient receiving appropriate treatment in a timely manner and is necessary to inform infection prevention interventions.
Rapid diagnostic tests for C. difficile
Una Health Ltd provides a range of market leading TECHLAB® diagnostic tests for C. difficile GDH and Toxin A/B detection. These tests are enzyme immunoassays in either 96-well plate or single membrane cassette formats. They provide rapid results that are simple to perform compared to cell cytotoxicity neutralisation assays and toxigenic culture.
The TECHLAB® range of kits largely comprise two formats that have the following benefits:
| CHEK™ 96-well plate-based enzyme immunoassay (EIA) | QUIK CHEK™ Single test membrane EIA technology in a cassette |
| – ELISA-based, 96-well plate format – Suitable for screening large numbers of samples – Results within 2 hours – Simple procedure – Automatable – Highly standardised | – Direct faecal specimen testing in a rapid assay format – Individual device – Membrane bound EIA technology – more sensitive than lateral flow tests – Suitable for smaller numbers of samples or for ‘out-of-workflow’ testing – Results within 30 minutes – Easy to interpret – No equipment needed – Highly specific and sensitive |
 |  |
Guidelines for detecting C. difficile
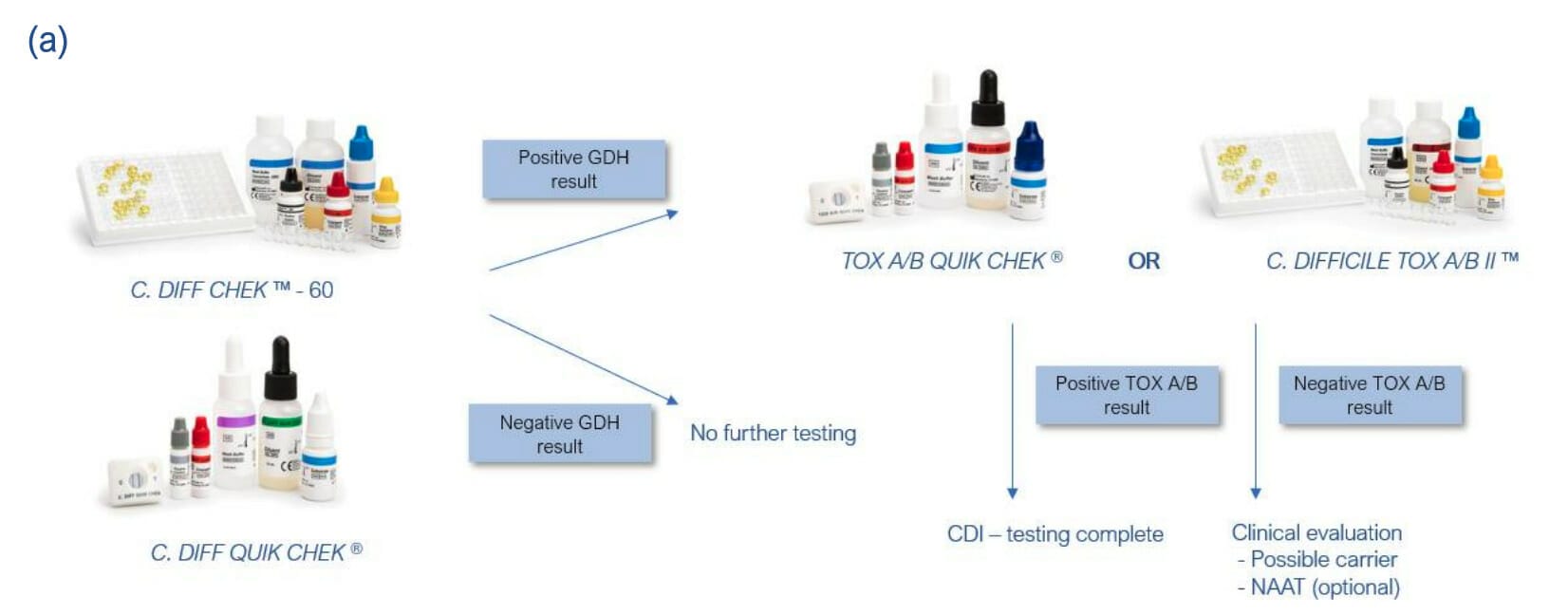
Not all strains of C. difficile produce toxins (non-toxigenic), therefore current UK guidelines advise that organisations adhere to a two-stage testing approach which consists of a test to screen for glutamate dehydrogenase (using EIA, NAAT, or PCR), followed by a toxin test.
This guidance is reflected by that of ESCMID, who revised their guidance for C. difficile infection (CDI) diagnosis in 20161.
No single test is suitable as a stand-alone test for CDI, but it is recommended to adopt a two test algorithm. The first test should be one that has high NPV (highly sensitive), which includes GDH EIA (C. DIFF CHEK™-60 or C. DIFF QUICK CHEK). The second reflex test should have high PPV (highly specific) so that all samples with a positive second test can be diagnosed as CDI. Toxin EIAs are recommended for this purpose as they detect free toxin and not just the gene. Samples with a first positive test but negative toxin A/B EIA need to be evaluated further (e.g. for low toxin producers or C. difficile carriage).
Recommended use of TECHLAB C. difficile tests based on algorithms by ESCMID for CDI testing: (a) GDH or NAAT – Tox A/B algorithm. (b) GDH and Tox A/B algorithm1.

Find out more about the available diagnostic tests for Clostridioides difficile from our latest white paper.

