Use of the CTX-M Multi NG Biotech Test for the detection of ESBL-producing Enterobacterales in a routine microbiology laboratory
Work completed by Anna Senior, Student/Medical Laboratory Assistant and Whiston Hospital Microbiology Department
Text written by Anna Senior and Carolyne Horner
Enterobacterales that produce extended-spectrum β-lactamases (ESBLs) are an important group of Gram-negative bacteria that cause a wide range of infections and may be resistant to multiple classes of antimicrobial agents.
ESBLs fall largely into three groups, TEM, SHV or CTX-M enzymes. In the UK, TEM and SHV were the prevalent enzymes pre-2000; however, in the early 2000s, CTX-M ESBL types emerged and became prevalent. Timely identification of these bacteria can inform appropriate antibiotic prescribing and infection prevention measures.
There are many different methods of detecting and confirming the presence of ESBL-producing bacteria. Methods used within the present work are the VITEK2® analyser (bioMerieux, France), disc synergy tests, the Rapid ESBL Screen kit 98022 (Rosco Diagnostica, Switzerland) and the CTX-M M NG Biotech test (NG Biotech, France).
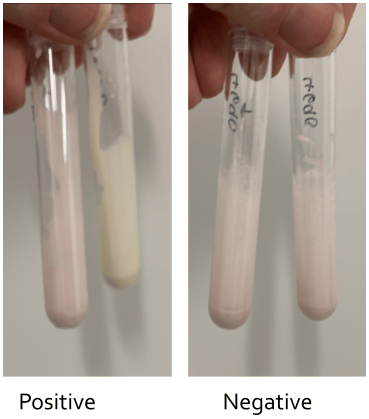
The Rapid ESBL Screen kit 98022 (Rosco Diagnostica, Switzerland) detects all types of ESBL and requires two tubes per isolate, one containing cefotaxime plus indicator and the other cefotaxime with tazobactam plus indicator. The test takes 30-40 minutes to set up, may take up to 2 hours to read the results. A control tube is required for each isolate. A positive result is indicated by a colour change from red to yellow (see Figure 1A).
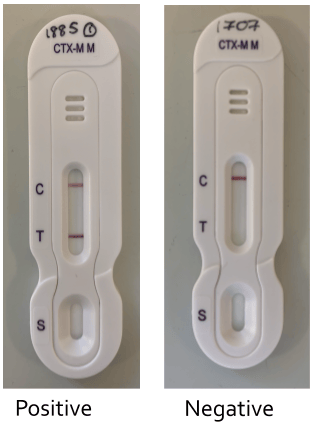
The CTX-M M NG Biotech test detects all five of the CTX-M groups (Groups 1, 2, 8, 9, 25), has both a control and test line within the cassette, takes 10 minutes to set up and a maximum of 15 minutes to run. Positive results are indicated by a coloured test line, plus a coloured control line; whereas a negative result is indicated by a coloured control line only. If there are no lines present, or only a single test line, the test is invalid.
At Whiston Hospital, detection and confirmation of ESBL-producing Enterobacterales may take up to three days. The current method to confirm ESBLs is considered expensive and results take up to two hours.
Aims
- To develop a more cost-effective protocol for confirmation of ESBLs
- To reduce turnaround time of results
Objectives
- To compare the NG Biotech CTX-M M test with the current method of ESBL confirmation
Eighty previously identified ESBL-producing Enterobacterales isolates were cultured from frozen storage. Isolates were identified and tested for antimicrobial susceptibility using the VITEK2® analyser. ESBL disc synergy tests were completed for each of the isolates:
- By VITEK2®, ESBL-producing bacteria were indicated by a MIC of >1mg/L for cefepime and ceftazidime and/or cefotaxime. Any indeterminate results were flagged for additional testing.
- By disc synergy tests, ESBL-producing bacteria were indicated by a >5mm zone diameter difference between the cefepime disc and the cefepime plus clavulanic acid disc and/or >5mm zone diameter difference between the cefpodoxime disc and the cefpodoxime plus clavulanic acid disc.
Twenty-three isolates were selected for further testing with the CTX-M M NG Biotech test and the Rosco ESBL Screen kit 98022:
- 15/23 (65%) isolates were positive using both the CTX-M M kit and the Rosco ESBL Screen
- 6/23 (26%) isolates were negative using the CTX-M M kit and positive by the Rosco ESBL Screen
- 2/23 (9%) isolates were negative using both the CTX-M M kit and the Rosco ESBL Screen
It is likely that the six isolates negative by CTX-M M kit and positive by Rosco ESBL screen belong to other ESBL types (TEM or SHV) rather than those belonging to the CTX-M family.
From this piece of work, a new protocol for the confirmation of ESBLs at Whiston Hospital is proposed. The VITEK2® and disc synergy tests will remain as ESBL screening methods; however, when either of these methods flag a potential ESBL, the CTX-M M NG Biotech cartridge test will be used in the first instance, providing results within 15 minutes. If a negative result arises from the CTX-M M cassette, the Rosco ESBL Screen will then be used to confirm the presence of an ESBL.
By implementing the CTM-M NG Biotech kit, the majority of ESBLs may be confirmed within 15 minutes and the price of each kit is less than the current method.
Acknowledgments
I would like to express my greatest gratitude to STHK Microbiology department at Whiston Hospital and Liverpool John Moores University for the advice and guidance offered to me throughout this project. In particular I would like to thank Lydia Newhall, Robert Price and Kerryanne Brown for their continued support in the lab. I would also like to thank my Mum and Dad for their support during the completion of my project and keeping me calm throughout.
Figure 1A. Results of ESBL testing using the Rapid ESBL Screen kit 98022.

Figure 1B. Results of ESBL testing using the CTX-M M NG Biotech kit.