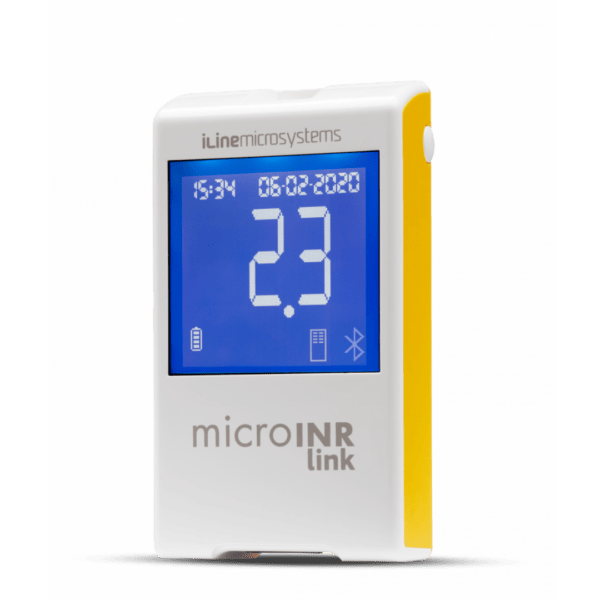
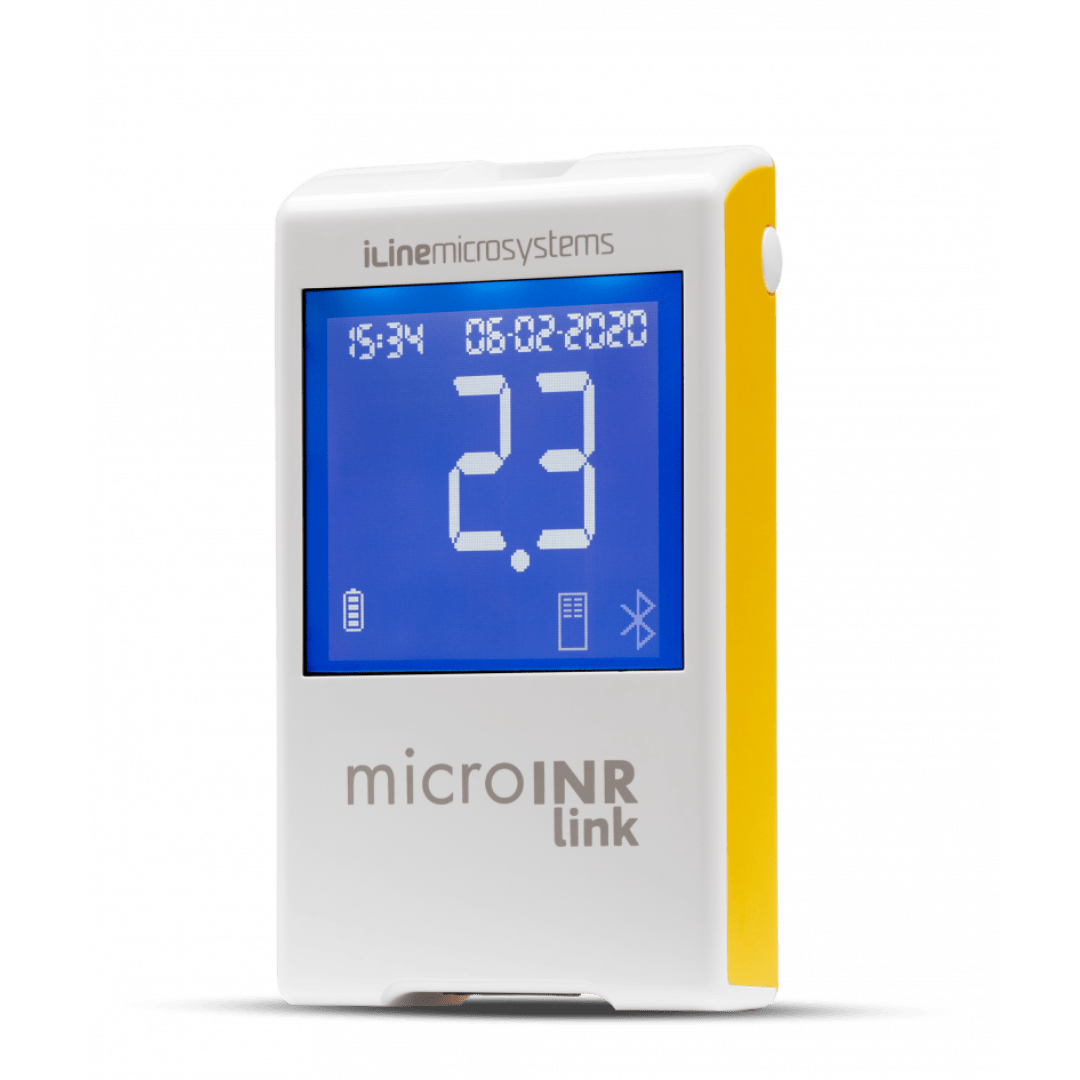
microINR Link Analyser
Leading the way in oral anti-coagulation therapy monitoring
Easy-to-use design, small & portable analyser for clinic settings or patient homes
The microINR System is an in vitro diagnostics medical device, intended to monitor oral anticoagulation therapy (OAT) with vitamin K antagonist drugs.
The system provides quantitative determination of prothrombin time (PT) in INR (International Normalized Ratio) units with fresh capillary blood performed by fingersticking.
Product Overview
The microINR System has been developed to fulfil the needs of all the existing oral anticoagulation therapy (OAT) monitoring models and it is intended for patient self-testing use, as well as for healthcare professionals at Point of Care settings.
The system provides quantitative determination of prothrombin time (PT) in INR (International Normalised Ratio) units with fresh capillary blood performed by fingersticking.
The microINR Link Meter combines all the advantages of the microINR Meter (fully automatic, minimum testing steps, low sample volume, multilevel QC strategy) with a built-in wireless Bluetooth® Low Energy 5.0 technology.
Bluetooth® connection with microINR is easy and convenient. By pairing microINR Link with a compatible device, the results will be automatically sent after its performance, keeping the testing steps set to the minimum.
Technical details

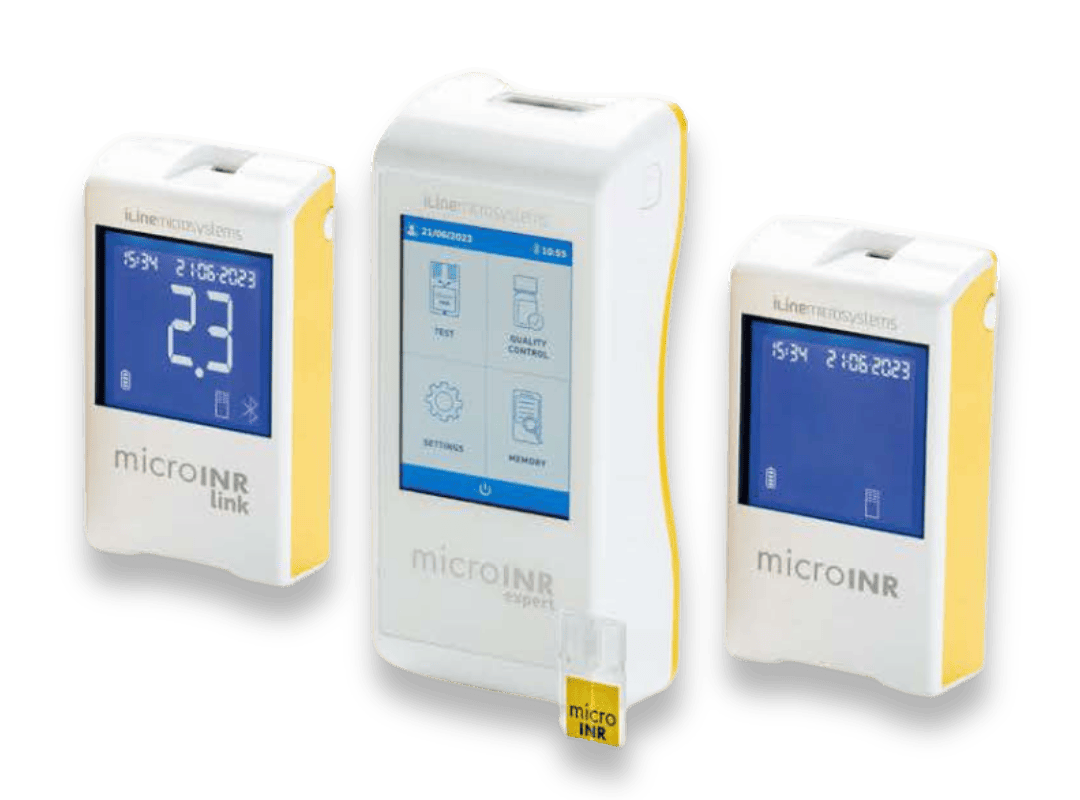
The microINR System refers to the reader (microINR Meter) and the analytic test strips (microINR Chips). There are three analysers with different connectivity options that all employ iLine’s innovative microfluidic and lab-on-a-chip technology, with the same test chip being used across all three devices.
This technology provides means to perform a biological test comprising sample application, reagent storage, mixing, detection and QC, all these areas embedded in a miniaturised chip using a minimum volume of blood sample. This innovative concept retains quality equivalents to the classical laboratory processes but, at the same time, provides the advantages of a user-friendly one-step assay.
Microfluidics Chips
The INR determination is achieved through sample flow monitoring along the micro-capillary channels of the Chip, following the activation of the coagulation cascade. The current IVD test mimics the conditions of “in vivo” hemostasis, also referred as “ex vivo”.
How to use the microINR system step by step