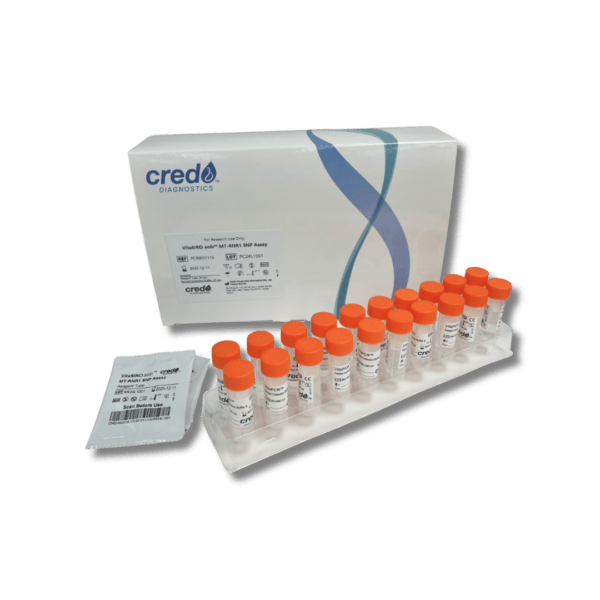
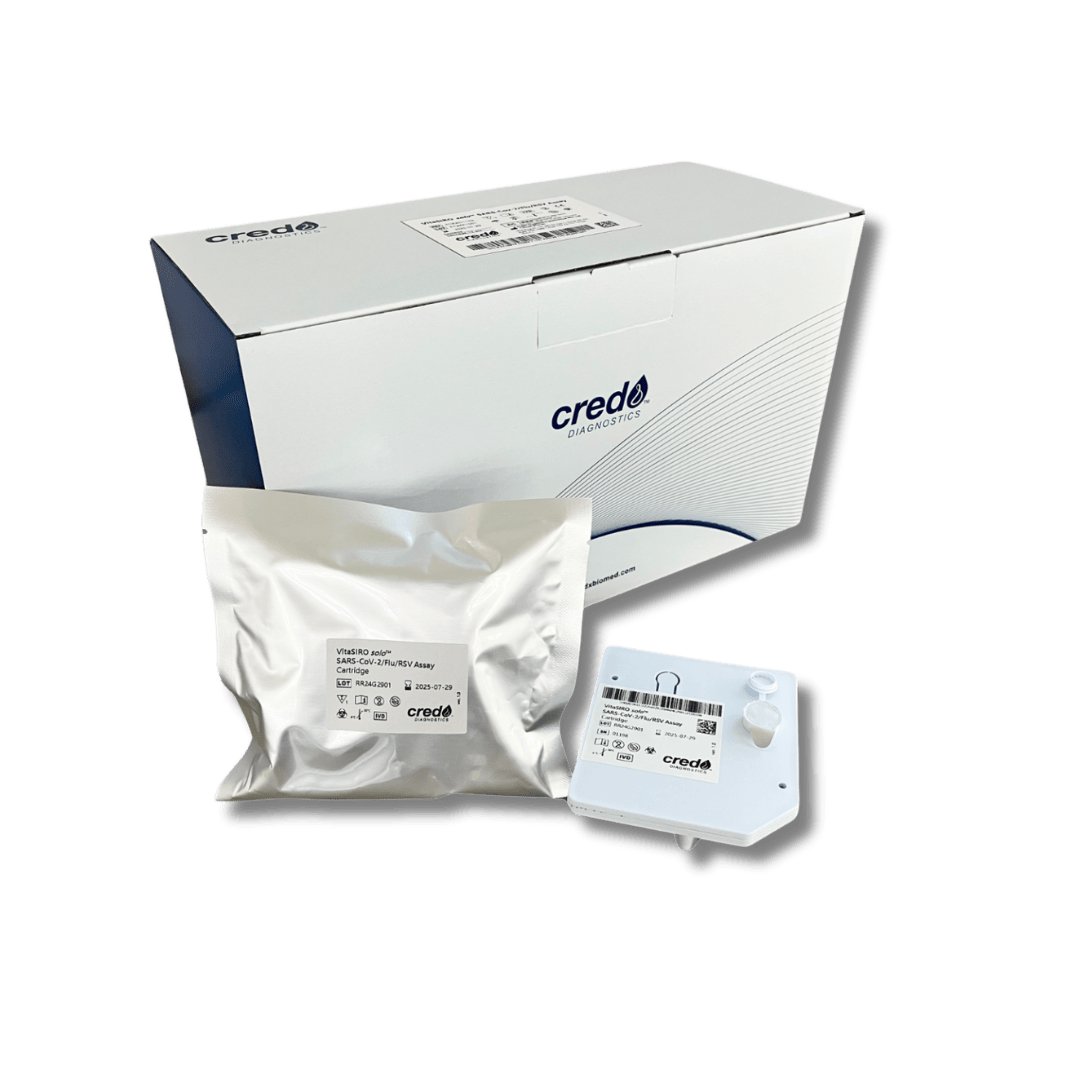
VitaSIRO solo™ MT-RNR1 SNP Assay
The Fastest MT-RNR1 Point of Care Test—Because Every Minute Matters
Aminoglycosides are a class of antibiotics commonly used to treat severe bacterial infections, but their use carries a significant risk of ototoxicity, especially in individuals with certain genetic predispositions. One such factor is the MT-RNR1 gene mutation, specifically the m.1555G variant, which greatly increases the risk of aminoglycoside-induced hearing loss. Early detection of this genetic variant is essential to prevent irreversible hearing damage in patients requiring aminoglycoside treatment.
Current genetic testing methods are limited by long processing times and the need for specialised laboratory facilities, highlighting the urgent need for a rapid, reliable, and accessible testing solution.
Product Overview
MT-RNR1 SNP assay The Fastest MT-RNR1 Point of Care Test—Because Every Minute Matters
A Single Nucleotide Polymorphism (SNP) in the MT-RNR1 gene—specifically m.1555A>G—is the primary genetic cause of Aminoglycoside-Induced Hearing Loss (AIHL). This mutation can lead to severe, irreversible ototoxicity when patients are treated with aminoglycoside antibiotics, which are commonly used to treat serious infections, especially in newborns and infants.
Clinical guidelines recommend that patients with this MT-RNR1 variant avoid aminoglycosides unless the risk of untreated infection outweighs the risk of hearing loss.¹ Rapid genetic testing enables personalised prescribing, helping prevent unnecessary and preventable hearing loss.
The Credo Vita Siro Solo: Fast, Accurate, and Reliable
The Credo Vita Siro Solo delivers MT-RNR1 variant results in just 20 minutes—the fastest test available—enabling timely treatment decisions within critical care windows.
Key Features:
- Non-invasive sample collection using a simple buccal swab
- Minimal hands-on time (<3 minutes) with results in 20 minutes
- Simple test procedure
- Room temperature storage for easy logistics and deployment
- High sensitivity and specificity for confident clinical decision-making
- Seamless connectivity with POCT1-A standards for integrated workflows
Make rapid, informed treatment decisions that protect patients from preventable hearing loss—with confidence and speed.
-
McDermott JH, Wolf J, Hoshitsuki K, Huddart R, Caudle KE, Whirl-Carrillo M, Steyger PS, Smith RJH, Cody N, Rodriguez-Antona C, Klein TE, Newman WG. Clinical Pharmacogenetics Implementation Consortium Guideline for the Use of Aminoglycosides Based on MT-RNR1 Genotype. Clin Pharmacol Ther. 2022 Feb;111(2):366-372. doi: 10.1002/cpt.2309. Epub 2021 Jun 20. PMID: 34032273; PMCID: PMC8613315.

This test delivers accurate results in just 20 minutes, enabling clinicians to quickly tailor treatments based on genetic profiles and prevent hearing loss. By bringing genetic testing to the point of care, this solution supports timely, informed decision-making and improves patient outcomes.
Aminoglycosides are a class of antibiotics commonly used to treat severe bacterial infections, but their use carries a significant risk of ototoxicity, especially in individuals with certain genetic predispositions. One such factor is the MT-RNR1 gene mutation, specifically the m.1555G variant, which greatly increases the risk of aminoglycoside-induced hearing loss. Early detection of this genetic variant is essential to prevent irreversible hearing damage in patients requiring aminoglycoside treatment.
Current genetic testing methods are limited by long processing times and the need for specialized laboratory facilities, highlighting the urgent need for a rapid, reliable, and accessible testing solution.
Diagnostics Procedure in 3 Steps:
Step 1
Collect sample
Step 2
Transfer the sample to the cartridge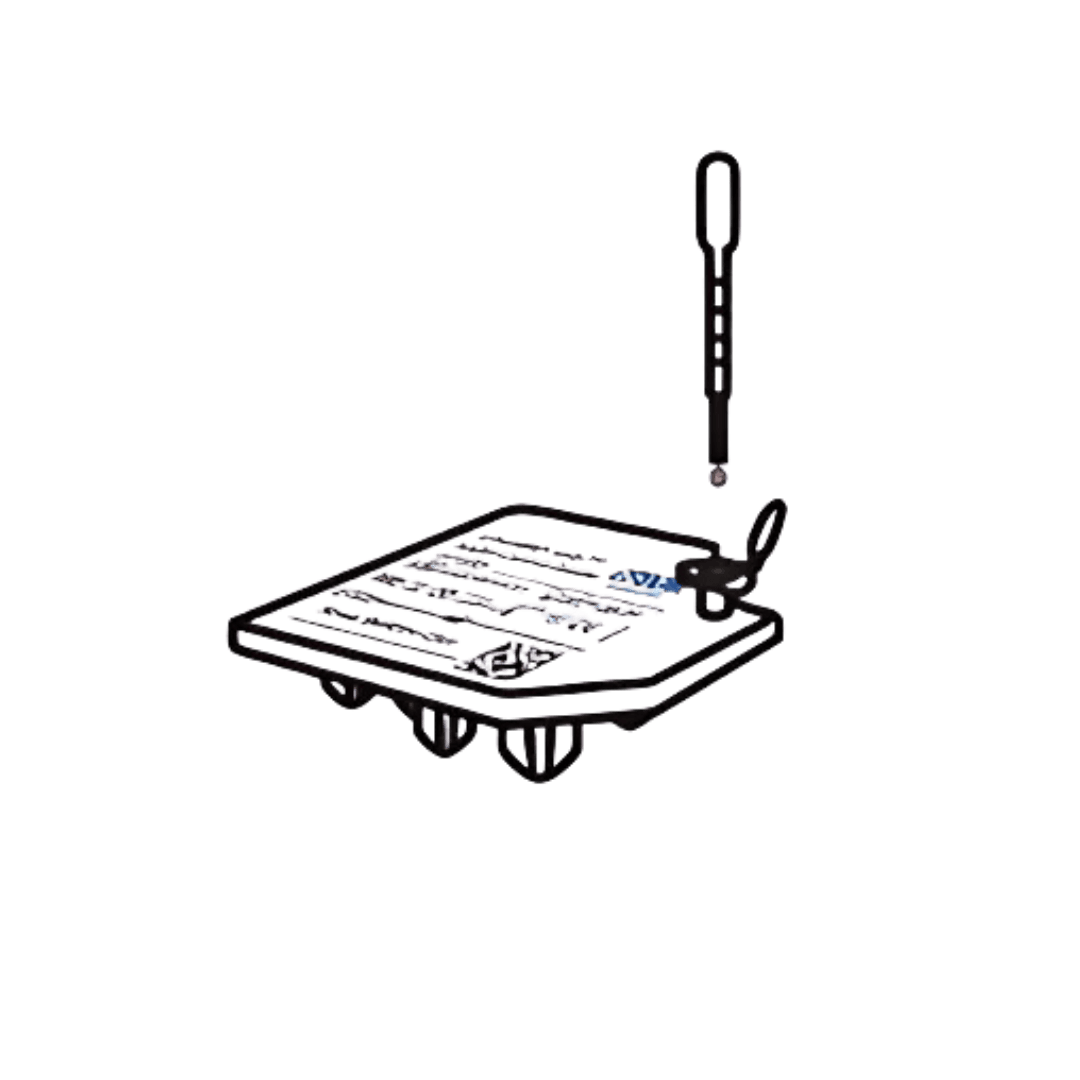
Step 3
Start run - results in 20 minutes