Understanding the prevalence of COVID-19: the role of rapid antibody testing
Detection of a serological response to SARS-CoV-2 has been integral to our understanding of virus transmission, development of infection, and recovery from COVID-19.
This blog post, an abbreviated version of an article published in Pathology in Practice (May 2021), describes how the application of rapid COVID-19 antibody detection contributed to surveillance that informed how the pandemic was progressing in England.
Author: Carolyne Horner
A significant health burden
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of COVID-19 (coronavirus disease 2019), an acute respiratory illness with a disease spectrum ranging from asymptomatic or mild symptoms (in most cases), to severe illness and life-threatening acute respiratory distress syndrome (ARDS).1
Since the WHO announced pandemic status (11 March 2020),2 SARS-CoV-2 has become a significant health burden; to date, there have been greater than 537 million cases and over 6.3 million deaths worldwide.3
Accurate diagnosis of COVID-19
Accurate diagnosis of COVID-19 is fundamental for appropriate, effective and timely patient care, and reduction of disease transmission in the community.4 Diagnostic strategies serve different purposes and comprise detection of SARS-CoV-2 RNA or antigen, or detection of antibody raised to SARS-CoV-2.
SARS-CoV-2 antigen tests are used to determine if a person is carrying the virus, either symptomatically or asymptomatically, and as such could be a source of infection to others. Antigen tests commonly involve detection of virus from a respiratory sample, such as a nasopharyngeal/oropharyngeal/nasal swab.5 The gold standard method of SARS-CoV-2 detection remains RT-PCR (reverse transcription polymerase chain reaction),1 a laboratory-based technique that commonly detects more than one target sequence, such as RNA encoding ORF1b, the spike or nucleocapsid proteins. 4,6
SARS-CoV-2 antibody tests (also known as COVID-19 antibody tests), on the other hand, are used to determine if an individual has encountered the virus previously and usually require a whole blood, plasma or serum sample to be taken after the onset of symptoms (>10 days).6 COVID-19 antibody tests commonly detect antibodies to the spike and/or nucleocapsid proteins.6
Unlike SARS-CoV-2 antigen detection, there is no gold standard method for COVID-19 antibody detection, meaning that it can be difficult to determine unbiased test sensitivity when evaluating new serology assays.7 Laboratory-based chemiluminescence (CLIA) or enzyme-linked immunosorbent assays (ELISA) are commonly used to detect SARS-CoV-2 antibodies, and may be used in combination as a composite reference.8 CLIA and ELISA methods have a high sample throughput, but usually require a venous blood sample, which has additional resource implications.
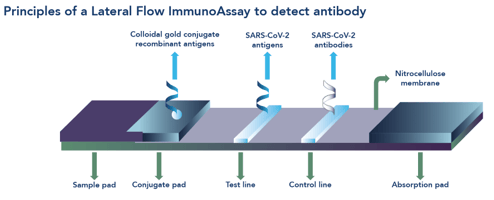
Unlike RT-PCR and CLIA or ELISA methods, lateral flow immunoassays (LFIAs) offer a rapid, point-of-care (POC) solution for COVID-19 diagnosis. LFIAs comprise a single use cassette (Box 1) that provide a result within 10-15 minutes without the need for a sample to be sent to the laboratory, thereby offering flexible, decentralised testing options for patient diagnosis and purposes of public health surveillance.9
The Fortress COVID-19 Total Antibody Device
The Fortress COVID-19 Total Antibody Device (Figure 1) is a single use, closed system, cassette-based in vitro immunochromatographic device for the qualitative detection of total antibodies against SARS-CoV-2 in human serum, plasma or whole blood specimens. The kit is intended for screening of patients suspected for infection with SARS-CoV-2, and as an aid in the diagnosis of COVID-19. The device comprises a control line and two test lines, one for detection of IgM and the other for detection of IgG.

Applications of COVID-19 antibody tests
Diagnostically, SARS-CoV-2 antibody tests are useful for patients with suspected COVID-19 who have Diagnostically, COVID-19 antibody tests are useful for patients with suspected infection with SARS-CoV-2 who have tested negative by RT-PCR; this may occur during the later stages of the disease when detectable virus may no longer be present.10 COVID-19 antibody tests are also useful for the diagnosis of asymptomatic/mild cases who may not have been tested for SARS-CoV-2 at all.
In terms of disease prevalence, antibody tests may be used for community screening of immune response to SARS-CoV-2. Monitoring the seroprevalence in the wider population may serve to 10,11
- identify/quantify the extent to which a population has been exposed to the virus,
- make predictions for how the pandemic may continue in the UK,
- characterise the epidemiology of the disease, such as identification of groups most at risk of infection of severe disease, and
- inform public health policy during the pandemic and longer term.
Inexpensive, point-of-care tests that require minimal training to complete and provide easy-to-interpret results, provide a source of inexpensive, widespread testing without direct laboratory support, enabling a much wider reach and impact of testing.
Rapid COVID-19 antibody tests: associated caveats
As with any diagnostic test, there are certain caveats associated with the application and widespread rollout of rapid antibody tests.
While rapid antibody tests offer a practical approach to identify individuals with previous exposure to SARS-CoV-2, further research is required to understand how antibody production maps to protection from infection. Currently there is no global standard for correlates of protection to SARS-CoV-2.12
Additionally, a negative result does not preclude the possibility of a previous encounter with SARS-CoV-2. There are multiple explanations for a negative result, not least the complexity of the human immune system. Various factors influence antibody production, such as when the blood sample was taken (LFIA are known to have variable sensitivity if used <21 days after symptom onset),13 age (extremes of age may not produce antibodies at the same speed/level as other age groups), and general health/comorbidities, (conditions causing suppression of the immune system).
Identifying the Landmark UK COVID-19 Surveillance Programme
In the UK, one landmark surveillance programme has been pivotal to our understanding of SARS-CoV-2, the disease spectrum it causes, and assessment of our response to natural infection and vaccination.
The REACT (REal time Assessment of Community Transmission) programme,14 commissioned by the Department of Health and Social Care, aimed to improve understanding of how the COVID pandemic was progressing across England.
The programme comprises two parts:
- REACT 1: monthly home-based antigen testing to determine current SARS-CoV-2 infection levels, and
- REACT 2: six weekly home-based antibody testing to determine how many people have been exposed to SARS-CoV-2 and have developed antibodies.
The REACT 2 arm of the programme required a reliable, rapid antibody POC test for use throughout the longevity of the study. To identify the highest performing LFIA available on the market, the REACT team extensively evaluated the suitability of a number of rapid antibody kits. Based on performance results, plus concordance between participant and observer finger prick result interpretation, and the availability of the kits on a large scale, the Fortress rapid antibody test was identified as the LFIA of choice for the REACT 2 study.
Further details of the REACT 2 programme and its seminal publications can be found in the reference list and resource box.
Rapid antibody testing supported the roadmap out of lockdown
The availability and systematic validation of rapid antibody LFIAs, for example Fortress Diagnostics COVID-19 Rapid Antibody test, available in the UK, enabled widespread population-based surveillance of the seroprevalence of SARS-CoV-2. Results from longitudinal studies, such as REACT 2, generated much needed high-quality evidence that informed how the pandemic was progressing in England. This evidence supported decision-making at crucial timepoints, including when it was safe to lift restrictions and enable the country to move out of lockdown.
See the full article here: Rapid antibody testing: a critical tool in understanding COVID-19 prevalence
Follow us on social networks to keep up to date with our latest news and blogs
References
1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2020.
2. Dhama K, Khan S, Tiwari R, et al. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev 2020; 33: 1–48.
3. Anon. Coronavirus Update (Live): Cases and Deaths from COVID-19 Virus Pandemic – Worldometer. Available at: https://www.worldometers.info/coronavirus/. Accessed April 6, 2021.
4. Kubina R, Dziedzic A. Molecular and serological tests for COVID-19. A comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics 2020; 10.
5. Anon. Test directory – FIND. Available at: https://www.finddx.org/test-directory/. Accessed November 4, 2021.
6. Caruana G, Croxatto A, Coste AT, et al. Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin Microbiol Infect 2020; 26: 1178–82.
7. Mulchandani R, Jones H E, Taylor-Phillips S, Shute J, Perry K JS et al. A of URTC (UK-R. Accuracy of UK Rapid Test Consortium (UK-RTC) “AbC-19 Rapid Test” for detection of previous SARS-CoV-2 infection in key workers: test accuracy study. BMJ 2020; 371.
8. Flower B, Brown JC, Simmons B, et al. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax 2020; 75: 1082–8.
9. Koczula, KM; Gallotta A. Lateral flow assays. Essays Biochem 2016; 60: 111–20.
10. Pallett S, Denny S, Patel A, et al. Point-of-care SARS‑CoV‑2 serological assays for enhanced case finding in a UK inpatient population. Sci Rep 2021; 11: 5860.
11. Ward H, Atchison C, Whitaker M, et al. Antibody prevalence for SARS-CoV-2 following the peak of the pandemic in England: REACT2 study in 100,000 adults. medRxiv 2020: 1–21.
12. Anon. What does immunogenicity mean in the context of COVID-19 vaccines? 2020. Available at: https://www.astrazeneca.com/what-science-can-do/topics/disease-understanding/what-does-immunogenicity-mean-in-the-context-of-covid-19-vaccines.html. Accessed April 6, 2021.
13. Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2020; 2020.
14. Anon. Real-time Assessment of Community Transmission (REACT) Study | Faculty of Medicine | Imperial College London. Available at: https://www.imperial.ac.uk/medicine/research-and-impact/groups/react-study/. Accessed April 6, 2021.
