New TECHLAB H. pylori verification against leading competitor
Work completed by Sandra Lees, SBMS for University Hospitals Plymouth, 2024
INTRODUCTION
The purpose of this verification study was to evaluate the performance of the TECHLAB Helicobacter Pylori CHEK™ ELISA (HP CHEK) kit compared to the current ThermoFisher OXOID™ Amplified IDEIA HpStAR ELISA kit used at University Hospitals Plymouth. By introducing this new kit, the department aims to streamline service without compromising the quality of results or affecting patient care.
METHOD
The study involved testing 115 samples, including positive, negative, and low-level positive samples, to determine the reproducibility of the TECHLAB kit’s results. Samples were tested using standard ELISA protocols on the Dynex DS2 instrument. Controls and Internal Quality Control (IQC Ridascreen) materials were used to ensure consistency.
RESULTS
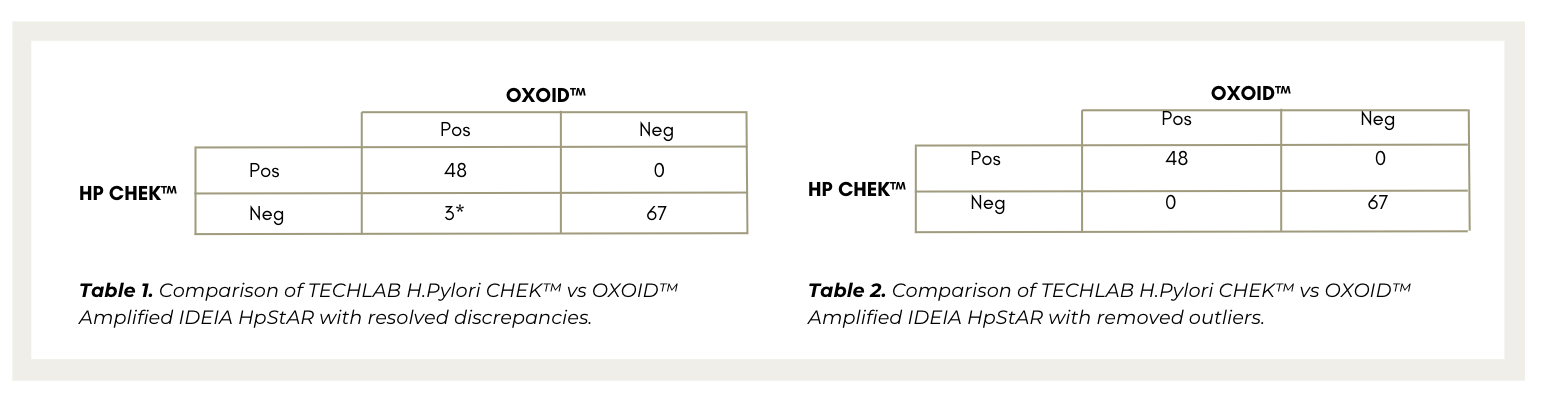
On initial testing there were 6 discrepant samples, of which 3 were resolved on repeat testing, resulting in a TECHLAB HP CHEK ™ sensitivity and specificity of 97.39%* and 100% respectively (Table 1.).
These 6 discrepant samples were negative with HP CHEK™ on both initial and repeat testing. Whereas, the OXOID™ assay saw 3 out of 6 samples change from positive to negative on repeat testing.
Further investigation of the 3 remaining discrepant samples showed that they were low-level positives, with the OXOID™ test been subjected to multiple freeze-thaw cycles and deemed outliers and removed. Upon removal, 100% concordance was achieved between HP CHEK™ and OXOID™ (Table 2.).
DISCUSSION
The TECHLAB HP CHEK™ ELISA kit outperformed ThermoFisher’s OXOID™ Amplified IDEIA HpStAR in several key areas. It achieved 100% concordance, whilst accurately identifying all known positives and negatives. One major advantage is the clarity of negative results; the TECHLAB kit consistently produced clearer and reproducible negatives, showing minimal OD variance across multiple runs, while the OXOID™ kit sometimes yielded borderline false positives.
In addition, the HP CHEK™ kit is 10 minutes faster, and improves workflows as there is no longer a need to make low level comments. Beyond its performance, adopting TECHLAB’s ELISA kit resulted in significant cost savings for the department whilst maintaining the current high standards of care without any additional requirements for service users.
