Helicobacter pylori testing – TECHLAB
The gold standard in enteric pathogen detection
Actionable results you can trust
TECHLAB’s H. pylori EIA solutions stand as the gold standard in enteric pathogen detection. The non-invasive Helicobacter pylori testing method ensures the identification of active H. pylori infection both pre- and post-treatment.
The H. pylori faecal antigen testing provides rapid results, facilitating timely intervention.
Product Overview
H. pylori faecal antigen testing from TECHLAB is a non-invasive EIA solution, the gold standard in enteric pathogen detection, helping to:
- Identify active Helicobacter pylori infection before and after treatment
- Obtain quick results to improve patient care
- Reduce sample rejection with flexible sample transport conditions
The H. pylori tests are available in 2 formats:
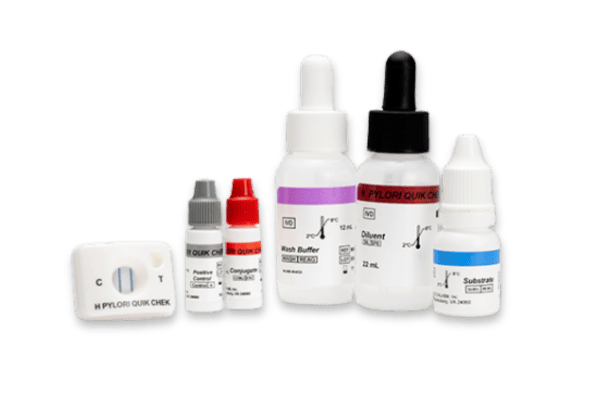
H. PYLORI QUIK CHEK ™
- Single test membrane EIA technology in a cassette
- Results in approximately 30 minutes
- Suitable for smaller numbers of samples or for ‘out-of-workflow’ testing
- Used with faecal specimens that are fresh, frozen, or in transport media
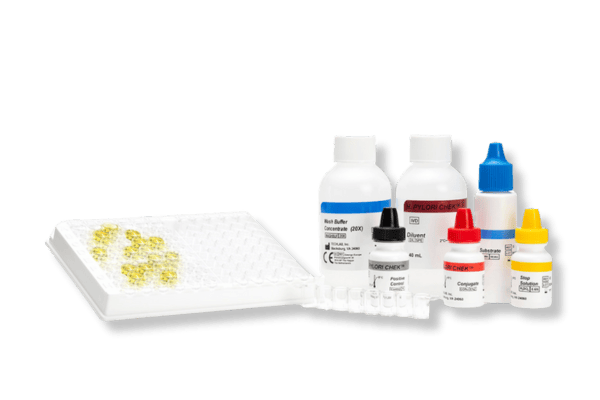
H. PYLORI CHEK™
- 96-well plate-based enzyme immunoassay
- Results in approximately 60 minutes
- Suitable for screening large numbers of samples
- Automatable*
- Used with faecal specimens that are fresh, frozen, or in transport media
*We can automate your ELISA solutions
Una Health have partnered with Aspect Scientific, to deliver the best-in-class ELISA automation for Enterics, Infectious Diseases, Immunology and Biochemistry assays.
Aspect Scientific’s market-leading DYNEX ELISA automation systems are capable of automating almost any ELISA assay. From single microplate readers and washers, up to fully automated 2, 4 & 12 plate systems, Aspect Scientific offer a range of solutions designed to fit your workload, throughput requirements and your budget.
Please get in contact with us at enquiries@unahealth.co.uk for more details on the automation options available.
What is the difference between a Quik Chek™ device and a lateral flow cassette?
H. pylori infection is the greatest known risk factor for gastric cancer.1 Although H. pylori infections are typically asymptomatic and do not require treatment, 20-25% of patients develop peptic ulcers or gastric cancer.
H. pylori infection affects nearly half of the global population, often asymptomatic and requiring no treatment. However, a minority may develop gastritis, and a fraction could face gastric ulcers or cancer risks. While the standard diagnosis involves endoscopy, current guidelines suggest this for alarm symptoms or older patients.
However, younger patients who do not have alarm symptoms may benefit from non-invasive tests, such as a faecal antigen test. These tests can also verify that H. pylori infection has been eradicated after treatment.
Resources:
- Wroblewski L.E., Peek R.M. Jr, Wilson K.T. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010. 23(4):713-39. doi: https://doi.org/10.1128/CMR.00011-10.
How it works
Principles of the QUIK CHEK™: single test membrane EIA technology in a cassette
The QUIK CHEK™ test is not a lateral flow assay. The QUIK CHEK™ test is a membrane-bound enzyme immunoassay (EIA) which uses antibodies that recognise specific antigens in human faecal samples.
The membrane device contains a small window in which the sample is added and a larger Reaction Window with vertical lines of immobilised antibodies. The test line (“T” or other label) contains antibodies against a specific antigen; the control line (“C”), contains anti-IgG antibodies. The Conjugate consists of antibodies to the specific antigen of interest coupled to horseradish peroxidase.
To complete the test, the procedure is completed as follows:
- A faecal specimen is added to a tube containing a mixture of Diluent and Conjugate.
- The diluted sample-conjugate mixture is added to the Sample Well and the device is allowed to incubate at room temperature for 15 minutes. During the incubation, the specific antigens in the sample bind to the antibody-peroxidase conjugate. The antigen-antibody complexes migrate through a filter pad to a membrane where they are captured by the immobilised anti-antigen antibodies in the line.
- After 15 minutes, the Reaction Window is subsequently washed with Wash Buffer, followed by the addition of Substrate.
- After another 10-minute incubation, the “T” reaction is examined visually for the appearance of a vertical blue line.
Interpretation of results
A blue line in the “Test” area indicates a positive test.
A positive “C” reaction, indicated by a vertical blue line (or three dots depending on the test), monitors/confirms that the sample and reagents were added correctly, the reagents were active at the time of performing the assay, and that the sample migrated properly through the Membrane Device. It also confirms the reactivity of the other reagents associated with the assay and that the results are valid.
Some QUEK CHEK™ membrane devices comprise a vertical line for the test result and another vertical line for the control, whereas others QUIK CHEK™ devices, such as the C. DIFF QUIK CHEK COMPLETE® for simultaneous detection of C. difficile GDH and toxins A and B, incorporate one vertical line for the GDH result (labelled Ag), three dots in the middle for the control, and another vertical line for the Toxin A/B detection (labelled TOX).
Kits typically contain (see specific kits for exact details of contents and safety advice):
- Membrane devices – each pouch contains 1 device
- Conjugate – Antibody to a specific antigen coupled to horseradish peroxidase in a buffered protein solution
- Diluent – Buffered protein solution with graduated dropper assembly
- Positive Control – specific antigen in a buffered protein solution
- Wash Buffer – Buffered solution with graduated dropper assembly
- Substrate – Solution containing tetramethylbenzidine
- Plastic, disposable graduated transfer pipettes
Principles of the CHEK™ test: a strip-based enzyme immunoassay (EIA) format
The test uses monoclonal and polyclonal antibodies to cell-surface antigens of the target analyte.
The Microassay Strips in the kit contain immobilised monoclonal antibodies against the antigen/s of interest, and the Conjugate consists of polyclonal antibodies against the antigens.
In the assay, an aliquot of a diluted faecal specimen is transferred to a microassay well. The immobilised monoclonal antibodies bind the antigen/s of interest if they are present. Upon addition, Conjugate then binds to the antigen/antibody complex. Any unbound materials are removed during the washing steps. Following the addition of Substrate, a yellow colour is detected due to the enzyme-antibody-antigen complexes that formed in the presence of antigens and conjugate.
Kits typically contain (see specific kits for exact details):
- Conjugate – Antibodies against specific faecal antigens in a buffered protein solution
- Diluent – Buffered protein solution. The Diluent is also to be used as the negative control solution
- Stop Solution – 0.6 N sulfuric acid
- Positive Control/s – specific antigens in a buffered protein solution with 0.02% thimerosal*
- Substrate – solution containing tetramethylbenzidine and peroxide
- Wash Buffer Concentrate – 20X concentrate containing phosphate buffered saline, detergent, and 0.2% thimerosal
- Microassay Plate – 12 strips, each consisting of 8 wells coated with antibodies to the antigen of interest (stored with desiccant)
- Additional Accessories: disposable plastic transfer pipettes, plastic adhesive sheets, Wash Solution label
Interpretation of results
In order for the results of the test to be valid, the negative control well should be colourless or have only a faint yellow colour and the positive control well should give a visible yellow colour.
A test sample is considered positive if it has an obvious yellow colour when compared to the negative control well. It may be less yellow or more yellow than the colour observed in the positive control well. A positive result indicates that the antigen/s of interest is/are present in the specimen.
A test sample is considered negative if the reaction is colourless or less yellow than the negative control well. A negative result indicates that the antigen/s is/are absent or the level is below the detection limit of the test.