TECHLAB® Diagnostics
Superior diagnosis of gastrointestinal disease
Rapid laboratory detection of a range of faecal antigens
TECHLAB® is a market leader of innovative, rapid, non-invasive diagnostic tests for gastrointestinal diseases, such as Clostridioides difficile, protozoan parasites, and other agents of foodborne illness. TECHLAB® diagnostic tests are also available for the detection of Helicobacter pylori and to differentiate between inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS).
Product Overview
Abdominal cramps, stomach pain and diarrhoea are common indicators of a gastrointestinal upset and can cause great discomfort and distress to a patient. These overlapping symptoms can be due to an infectious agents or a non-infectious cause.
The TECHLAB® range of kits are ideal to determine the common causes of diarrhoeal illness in different clinical scenarios:
Infectious causes:
- Clostridioides difficile: GDH (glutamate dehydrogenase) and/or toxin A/B detection
- Foodborne illness: Shiga toxin-producing Escherichia coli (STEC), Campylobacter species, Clostridium perfringens enterotoxin detection
- Faecal parasite detection: Giardia lamblia, Cryptosporidium, Entamoeba histolytica
- Helicobacter pylori: gastritis, gastric ulcers or gastric cancer
Non-infectious causes:
- Faecal lactoferrin – for accurate differentiation between inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS).
There are two formats of TECHLAB® kit:
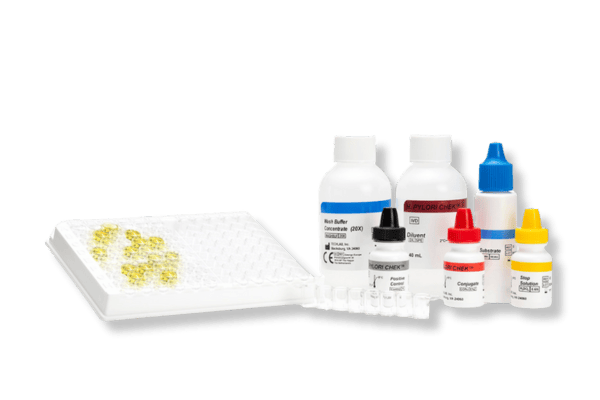
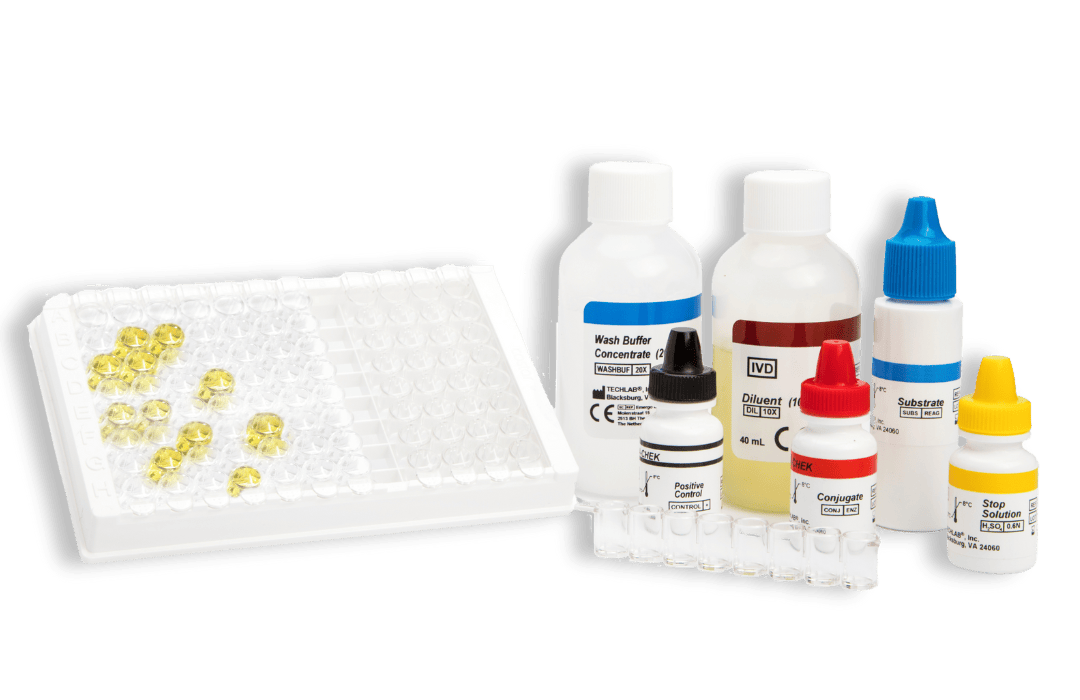
- CHEK: 96-well plate-based enzyme immunoassay (EIA) format
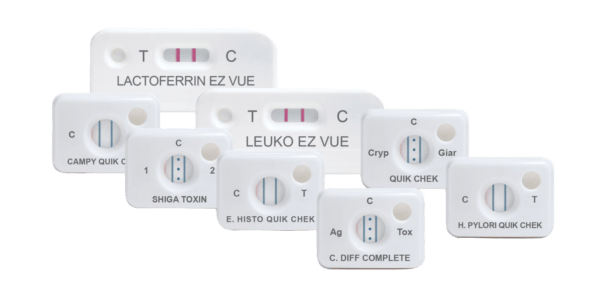
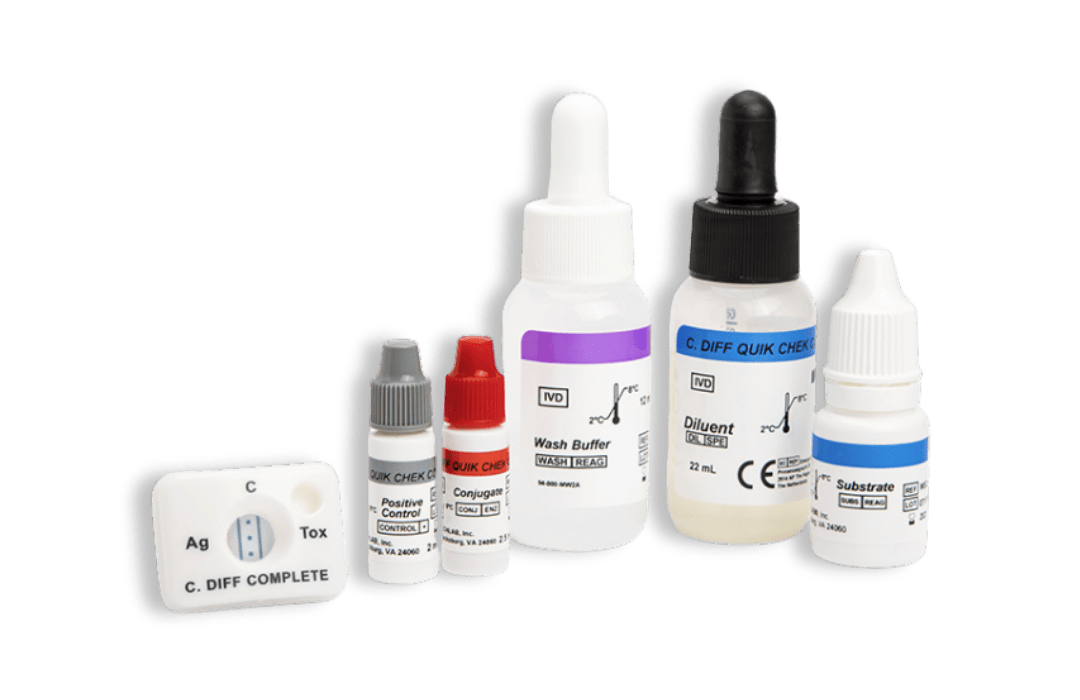
- QUIK CHEK: single test membrane EIA technology in a cassette
What is the difference between a Quik Chek™ device and a lateral flow cassette?
For more details and technical briefings, please refer to our Resources section.
The TECHLAB® facilities have ISO 13485 certification and the kits are CE marked.
We can automate your ELISA solutions
Una Health have partnered with Aspect Scientific, to deliver the best-in-class ELISA automation for Enterics, Infectious Diseases, Immunology and Biochemistry assays.
Aspect Scientific’s market-leading DYNEX ELISA automation systems are capable of automating almost any ELISA assay. From single microplate readers and washers, up to fully automated 2, 4 & 12 plate systems, Aspect Scientific offer a range of solutions designed to fit your workload, throughput requirements and your budget.
Please get in contact with us at enquiries@unahealth.co.uk for more details on the automation options available.
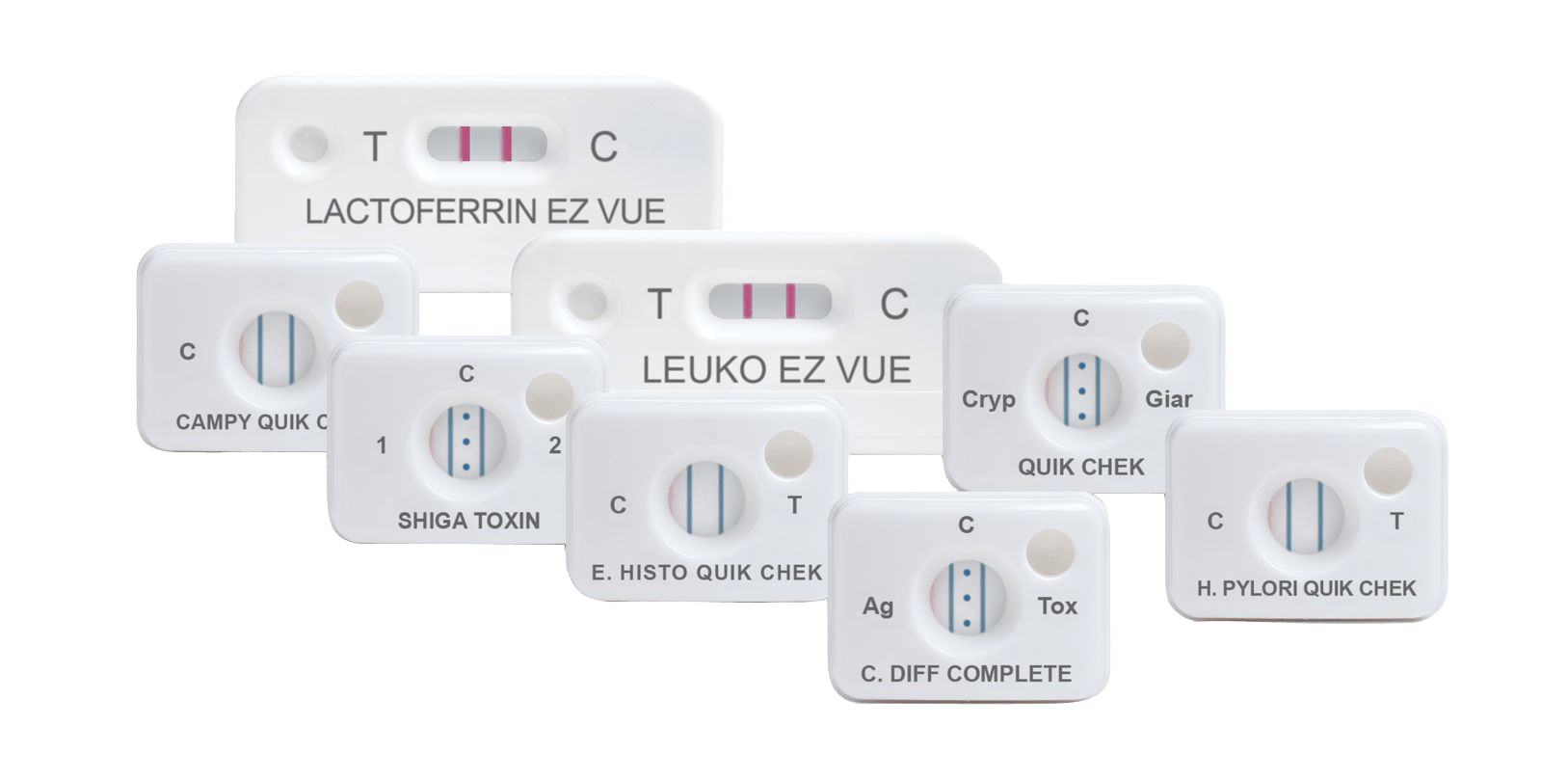
How it works
Principles of the QUIK CHEK™: single test membrane EIA technology in a cassette
The QUIK CHEK™ test is not a lateral flow assay. The QUIK CHEK™ test is a membrane-bound enzyme immunoassay (EIA) which uses antibodies that recognise specific antigens in human faecal samples.
The membrane device contains a small window in which the sample is added and a larger Reaction Window with vertical lines of immobilised antibodies. The test line (“T” or other label) contains antibodies against a specific antigen; the control line (“C”), contains anti-IgG antibodies. The Conjugate consists of antibodies to the specific antigen of interest coupled to horseradish peroxidase.
To complete the test, the procedure is completed as follows:
- A faecal specimen is added to a tube containing a mixture of Diluent and Conjugate.
- The diluted sample-conjugate mixture is added to the Sample Well and the device is allowed to incubate at room temperature for 15 minutes. During the incubation, the specific antigens in the sample bind to the antibody-peroxidase conjugate. The antigen-antibody complexes migrate through a filter pad to a membrane where they are captured by the immobilised anti-antigen antibodies in the line.
- After 15 minutes, the Reaction Window is subsequently washed with Wash Buffer, followed by the addition of Substrate.
- After another 10-minute incubation, the “T” reaction is examined visually for the appearance of a vertical blue line.
Interpretation of results
A blue line in the “Test” area indicates a positive test.
A positive “C” reaction, indicated by a vertical blue line (or three dots depending on the test), monitors/confirms that the sample and reagents were added correctly, the reagents were active at the time of performing the assay, and that the sample migrated properly through the Membrane Device. It also confirms the reactivity of the other reagents associated with the assay and that the results are valid.
Some QUEK CHEK™ membrane devices comprise a vertical line for the test result and another vertical line for the control, whereas others QUIK CHEK™ devices, such as the C. DIFF QUIK CHEK COMPLETE® for simultaneous detection of C. difficile GDH and toxins A and B, incorporate one vertical line for the GDH result (labelled Ag), three dots in the middle for the control, and another vertical line for the Toxin A/B detection (labelled TOX).
Kits typically contain (see specific kits for exact details of contents and safety advice):
- Membrane devices – each pouch contains 1 device
- Conjugate – Antibody to a specific antigen coupled to horseradish peroxidase in a buffered protein solution
- Diluent – Buffered protein solution with graduated dropper assembly
- Positive Control – specific antigen in a buffered protein solution
- Wash Buffer – Buffered solution with graduated dropper assembly
- Substrate – Solution containing tetramethylbenzidine
- Plastic, disposable graduated transfer pipettes
What is the difference between a Quik Chek™ device and a lateral flow cassette?
Principles of the CHEK™ test: a strip-based enzyme immunoassay (EIA) format
The test uses monoclonal and polyclonal antibodies to cell-surface antigens of the target analyte.
The Microassay Strips in the kit contain immobilised monoclonal antibodies against the antigen/s of interest, and the Conjugate consists of polyclonal antibodies against the antigens.
In the assay, an aliquot of a diluted faecal specimen is transferred to a microassay well. The immobilised monoclonal antibodies bind the antigen/s of interest if they are present. Upon addition, Conjugate then binds to the antigen/antibody complex. Any unbound materials are removed during the washing steps. Following the addition of Substrate, a yellow colour is detected due to the enzyme-antibody-antigen complexes that formed in the presence of antigens and conjugate.
Kits typically contain (see specific kits for exact details):
- Conjugate – Antibodies against specific faecal antigens in a buffered protein solution
- Diluent – Buffered protein solution. The Diluent is also to be used as the negative control solution
- Stop Solution – 0.6 N sulfuric acid
- Positive Control/s – specific antigens in a buffered protein solution with 0.02% thimerosal*
- Substrate – solution containing tetramethylbenzidine and peroxide
- Wash Buffer Concentrate – 20X concentrate containing phosphate buffered saline, detergent, and 0.2% thimerosal
- Microassay Plate – 12 strips, each consisting of 8 wells coated with antibodies to the antigen of interest (stored with desiccant)
- Additional Accessories: disposable plastic transfer pipettes, plastic adhesive sheets, Wash Solution label
Interpretation of results
In order for the results of the test to be valid, the negative control well should be colourless or have only a faint yellow colour and the positive control well should give a visible yellow colour.
A test sample is considered positive if it has an obvious yellow colour when compared to the negative control well. It may be less yellow or more yellow than the colour observed in the positive control well. A positive result indicates that the antigen/s of interest is/are present in the specimen.
A test sample is considered negative if the reaction is colourless or less yellow than the negative control well. A negative result indicates that the antigen/s is/are absent or the level is below the detection limit of the test.
How the TECHLAB® kits help
Gastrointestinal diseases can cause great discomfort and distress to a patient.
Rapid and accurate diagnosis of gastrointestinal disease is central to a patient receiving appropriate treatment and symptomatic relief in a timely manner and is necessary to inform public health actions.
The TECHLAB® range offers the following tests from faecal samples:
• Clostridioides difficile: GDH (glutamate dehydrogenase) and/or toxin A/B detection
• Foodborne bacterial illness:
o Campylobacter species
o Shiga toxin-producing Escherichia coli (STEC)
o Clostridium perfringens enterotoxin detection
• Faecal parasite detection: Giardia lamblia, Cryptosporidium, Entameoba histolytica
• Helicobacter pylori
• Faecal lactoferrin
Technical Briefings on the TECHLAB® product range
Superior diagnosis of gastrointestinal disease (Technical Briefing)
Diagnostic tests for Clostridioides difficile (White paper)
Diagnosis of gastrointestinal bacteria (Technical Briefing)
Diagnosis of common faecal parasites (Technical Briefing)
Diagnosis of inflammatory intestinal diseases (Technical Briefing)