Product Overview
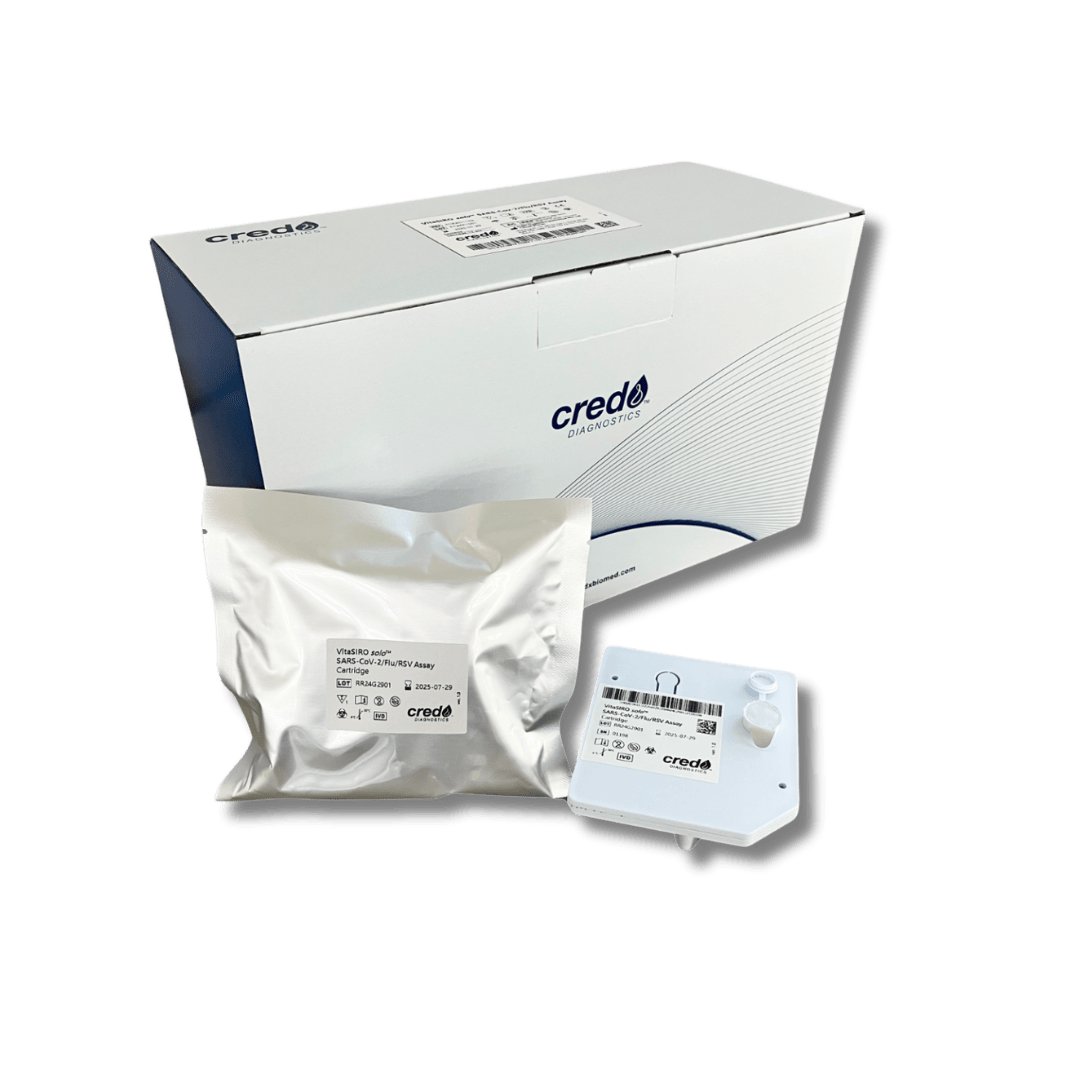
VitaSIRO solo™ is a compact, portable molecular diagnostics device that enables nucleic acid testing directly at the point of care. Using a patented cartridge system, it integrates nucleic acid extraction and PCR amplification into one workflow, providing high-quality rapid results with minimal hands-on time.
Designed for flexibility, VitaSIRO solo™ can be used to detect multiple genetic targets from a variety of sample types including saliva, blood, urine, faeces, and UTM/VTM swabs. Its research applications currently include assays for SARS-CoV-2, Influenza A/B, and RSV*, as well as an MT-RNR1 genetic assay for investigating neonatal susceptibility to aminoglycoside-induced hearing loss (AIHL).
With its simple test procedure, 3.6 kg design, 7-inch touchscreen, and POCT1-A connectivity, VitaSIRO solo™ supports decentralised research workflows, enabling efficient, scalable studies without reliance on central laboratories.
*Pending CTDA approval

VitaSIRO solo™ simplifies advanced molecular diagnostics by combining nucleic acid extraction and real-time PCR in a single cartridge-based workflow. A sample – whether from saliva, urine, blood, faeces, or UTM/VTM – is loaded into the cartridge, which the system then processes automatically. The device delivers accurate, lab-quality results directly on its integrated touchscreen interface. With the ability to detect up to six genetic targets at once, and with seamless POCT1-A connectivity for data transfer, VitaSIRO solo™ ensures precise and reliable diagnostics with minimal operator input.
By integrating real-time PCR into a compact, cartridge-based platform, VitaSIRO solo™ enables decentralised molecular diagnostics without compromising analytical performance. The system supports multiplex detection of up to six genetic targets across diverse sample types, with validated assays for both respiratory pathogens and MT-RNR1 genetic screening. Its portable 3.6 kg design, combined with POCT1-A connectivity, facilitates deployment in hospitals, clinics and community settings. This allows rapid turnaround of clinically actionable results, reducing diagnostic bottlenecks in centralised laboratories, improving infection control measures, and supporting early intervention strategies to enhance patient safety.
Diagnostics Procedure in 3 Steps:
Step 1
Collect Sample
Step 2
Transfer the sample to the cartridge
Step 3
Start Run - results available in under one hour (timing may vary by assay)