
Syphilis Serology: how does the Fortress Diagnostics TPHA (Syphilis) perform?
Work completed by Graham Pickard, Senior Biomedical Scientist and Paul Lane, Senior Biomedical Scientist, Department of Infection and Immunity, Charing Cross Hospital, Imperial College Healthcare NHS Trust
Report written by Carolyne Horner: 29th July 2022. Updated by Una Health 12th August 2022
Background
Syphilis is the fourth most frequent cause of a new diagnosis of sexually transmitted infection (STI) in England and prevalence is increasing; the number of new diagnoses of syphilis for both sexes in England has increased year-on-year, from 2901 cases in 2011 to 8011 cases in 2019 (UKHSA STI annual data tables).
Not everyone infected with syphilis will exhibit symptoms and any symptoms that are present may disappear; infection remains lifelong unless treated. Accurate diagnosis of syphilis is critical to prevent spread of infection, reduce complications associated with infection during pregnancy, and minimise long-term sequelae, such as effects on cardiovascular function and neurosyphilis.
Unlike many other bacteria, Treponema pallidum, the causative agent of syphilis, is not easy to grow in the laboratory and diagnosis relies on detection of T. pallidum antibodies. The UK Standards for Microbiology Investigations (SMI V.44.2.1) recommends screening for the presence of treponemal IgM and IgG using an EIA (enzyme immunoassay) or CLIA (chemiluminescent immunoassay). Any samples that test positive or equivocal by the primary screen require secondary testing using a secondary serology method, such as a Treponema pallidum particle agglutination assay (TPPA), Treponema pallidum haemagglutination assay (TPHA), Treponema pallidum latex agglutination test automated turbidimetric assay (TPLA), and rapid plasma regain (RPR). The difference between the TPPA/TPHA/TPLA is based on the cells used for agglutination: TPPA uses gelatin particles; TPHA red blood cells, and TPLA latex particles.
One commonly used TPPA is the Serodia® TPPA from Fujirebio, Japan. Fujirebio have reported that they will no longer supply the Serodia TPPA kits to the UK by 31 December 2023 and laboratories that currently use this test will need to source a suitable replacement. The TPHA from Fortress Diagnostics is an alternative method for the detection of antibodies to T. pallidum in human serum and plasma. For use either qualitatively or quantitatively, results are ready within 45-60 minutes.
The team in the Department of Infection and Immunity, Charing Cross Hospital wanted to compare the use of the Fortress TPHA against the Serodia® TPPA for use in their laboratory.
Results
Using the Abbott Alinity Syphilis TP assay as a primary screen, 59 serum samples with reactive or equivocal results were tested using SERODIA®-TPPA (current method) and Fortress TPHA (evaluation method) and subsequently (when required) by a reference lab for confirmation.
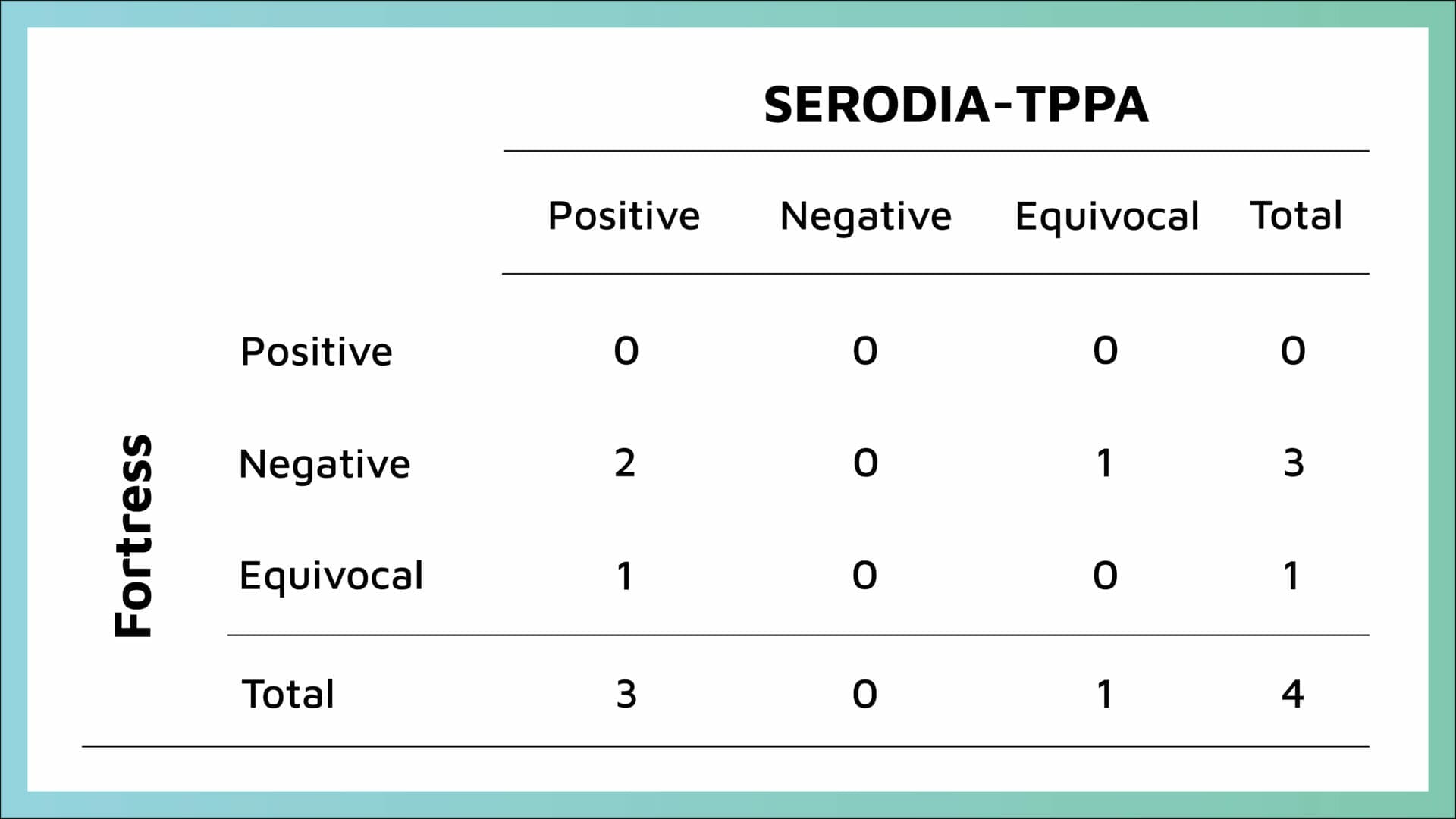
Five samples had equivocal results by TPPA and were excluded from sensitivity/specificity analysis. According to TPHA instructions for use, samples that are repeatedly equivocal should be considered positive. To expand on this, of the five TPPA Equivocal samples three were Negative on TPHA of which the reference lab confirmed as Negative and for the other two TPPA Equivocal samples the TPHA returned an Equivocal and a weak positive (these two samples were not referred to the reference lab).
With the remaining 54 samples which gave a POS or NEG using the SERODIA-TPPA, the Fortress TPHA had a sensitivity of 94.6% and 100% specificity (Table 1).
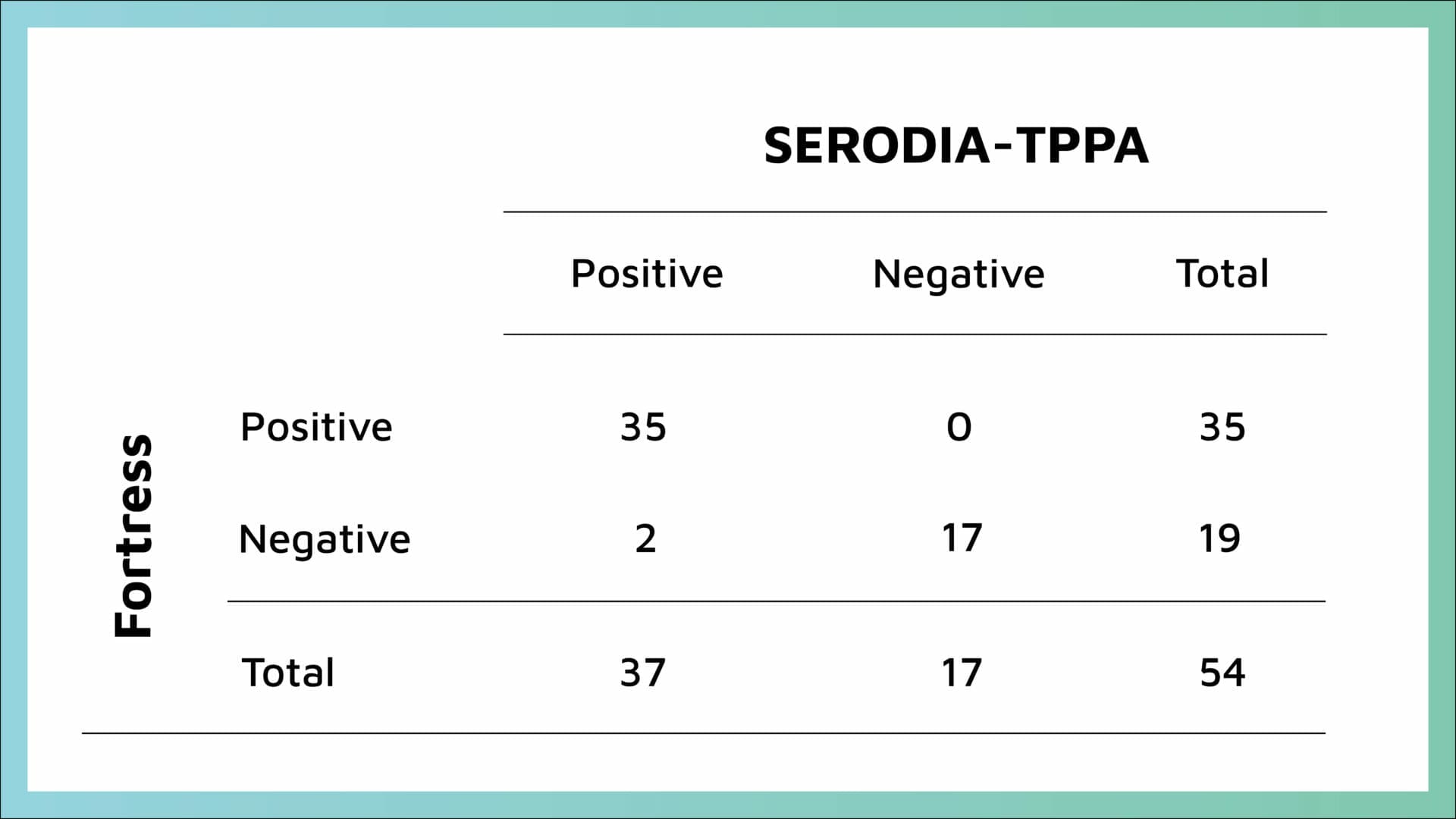
Four discrepant samples were sent to the Reference Laboratory for confirmation with results returned (Table 2):
- One sample tested equivocal by TPPA and tested negative by TPHA this sample was confirmed as negative by the reference laboratory
- One sample tested positive by TPPA and was equivocal by TPHA – this sample was confirmed as positive. This sample was not considered a discrepant result between methods as if interpreted correctly by the manufacturer’s instructions for use, repeatable equivocal TPHA result should be considered positive.
- Two samples tested positive by TPPA and were negative by TPHA – one was confirmed positive by the reference laboratory and the second was confirmed as negative by the reference laboratory
TPPA is known to be the more sensitive of the two assays. According to SMI V.44.2.1: “TPPA is one of the first tests to become reactive in primary syphilis, and is reactive earlier than TPHA.”
Laboratory staff at Charing Cross reported that the Fortress TPHA was easy to use and the results were easy to interpret and the test had a faster turnaround than the TPPA.
Following the evaluation, the laboratory at Charing Cross Hospital have switched their secondary syphilis serology testing to the Fortress TPHA in good time before availability of the SERODIA-TPPA becomes limited.

Case Study available for download:
Syphilis Serology: how does the Fortress Diagnostics TPHA (Syphilis) perform?
Share

Find out more about TPHA (Syphilis) Test from Fortress Diagnostics
