C. difficile GDH Testing – TECHLAB®
The gold standard in enteric pathogen detection
Clostridioides difficile (C. difficile) is the major cause of nosocomial diarrhoea and pseudomembranous colitis. The bacterium produces spores and infection usually occurs when antibiotics alter or kill the normal gut microflora, allowing the germination of spores into vegetative cells.
Product Overview
The glutamate dehydrogenase (GDH) of C. difficile is a good antigen marker for the organism in faeces, because it is produced in high amounts by all strains, toxigenic or non-toxigenic. C. difficile range of tests from TECHLAB help with the detection of the clostridioides difficile antigen in faecal specimens.
The C. difficile testing options from TECHLAB are available in 2 formats:
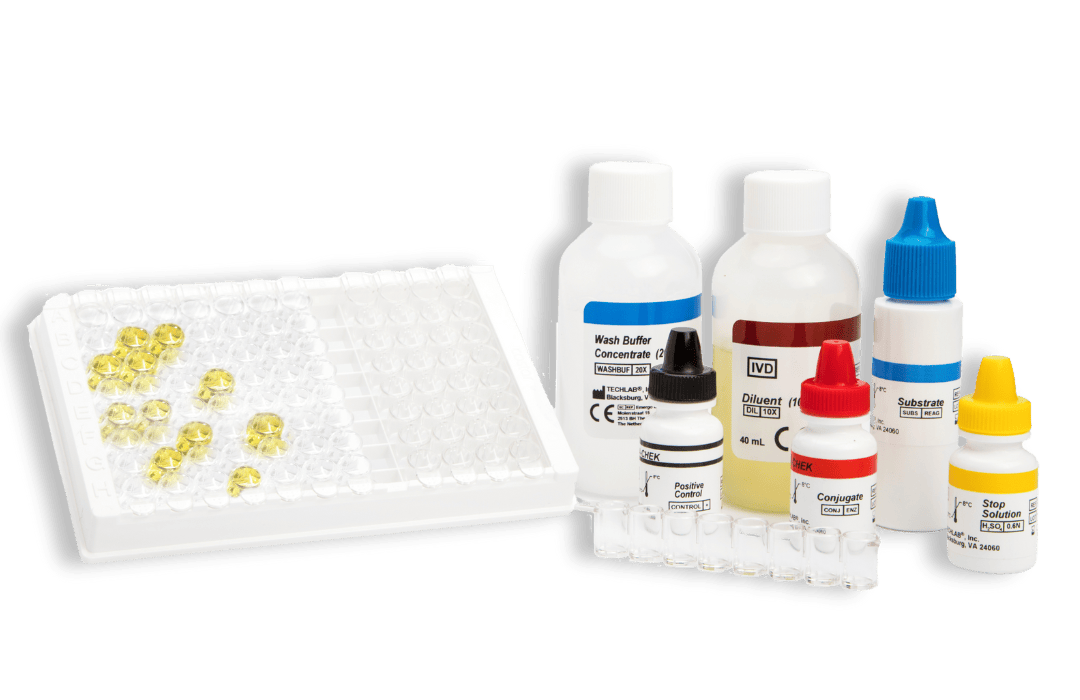
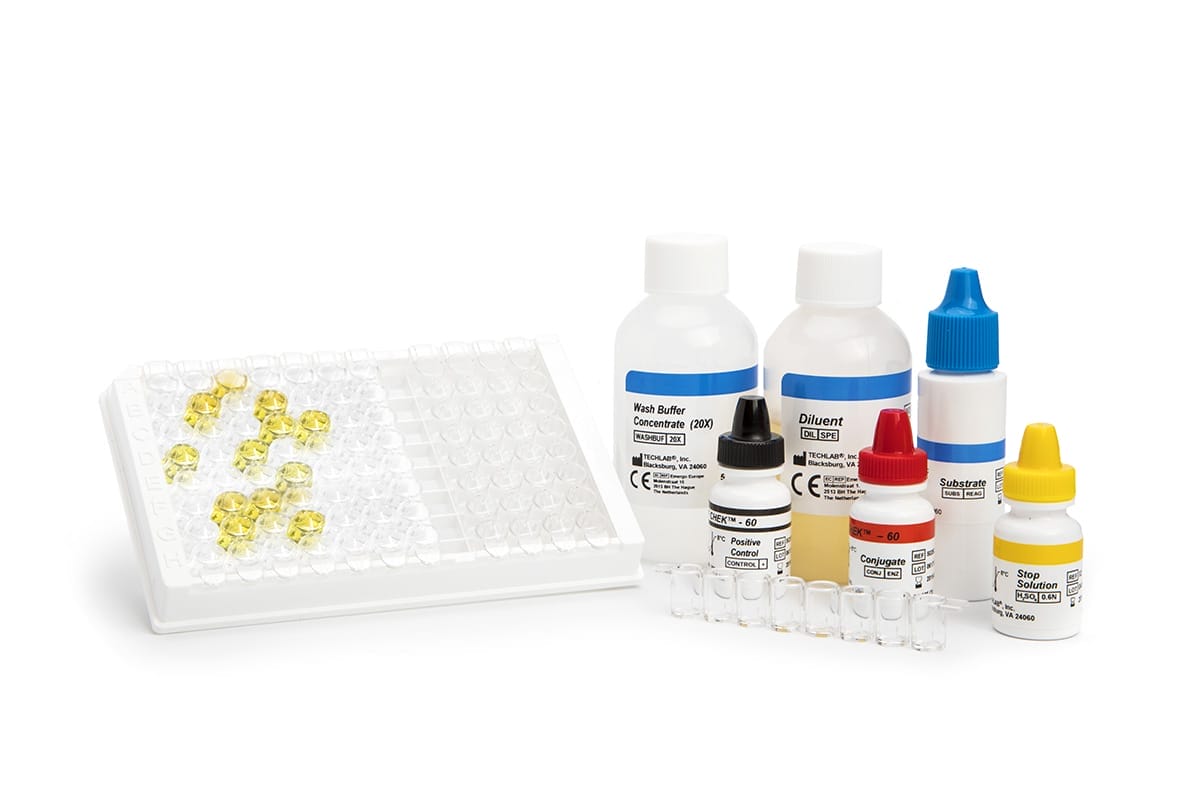
C. DIFF CHEK ™ – 60
TL5025
- Gold standard ELISA assay for the detection of glutamate dehydrogenase (GDH)
- Results in approx 60 minutes
- Suitable for screening large numbers of samples
- Automatable*
- Sensitivity and Negative Predictive Value (NPV) of GDH are equivalent to toxigenic culture
- A positive result in the test, which is highly specific for the glutamate dehydrogenase of C. difficile, confirms the presence of this organism in a fecal specimen; a negative result indicates the absence of the organism. A positive result should be followed by a toxin-specific test to confirm the presence of toxigenic C. difficile.
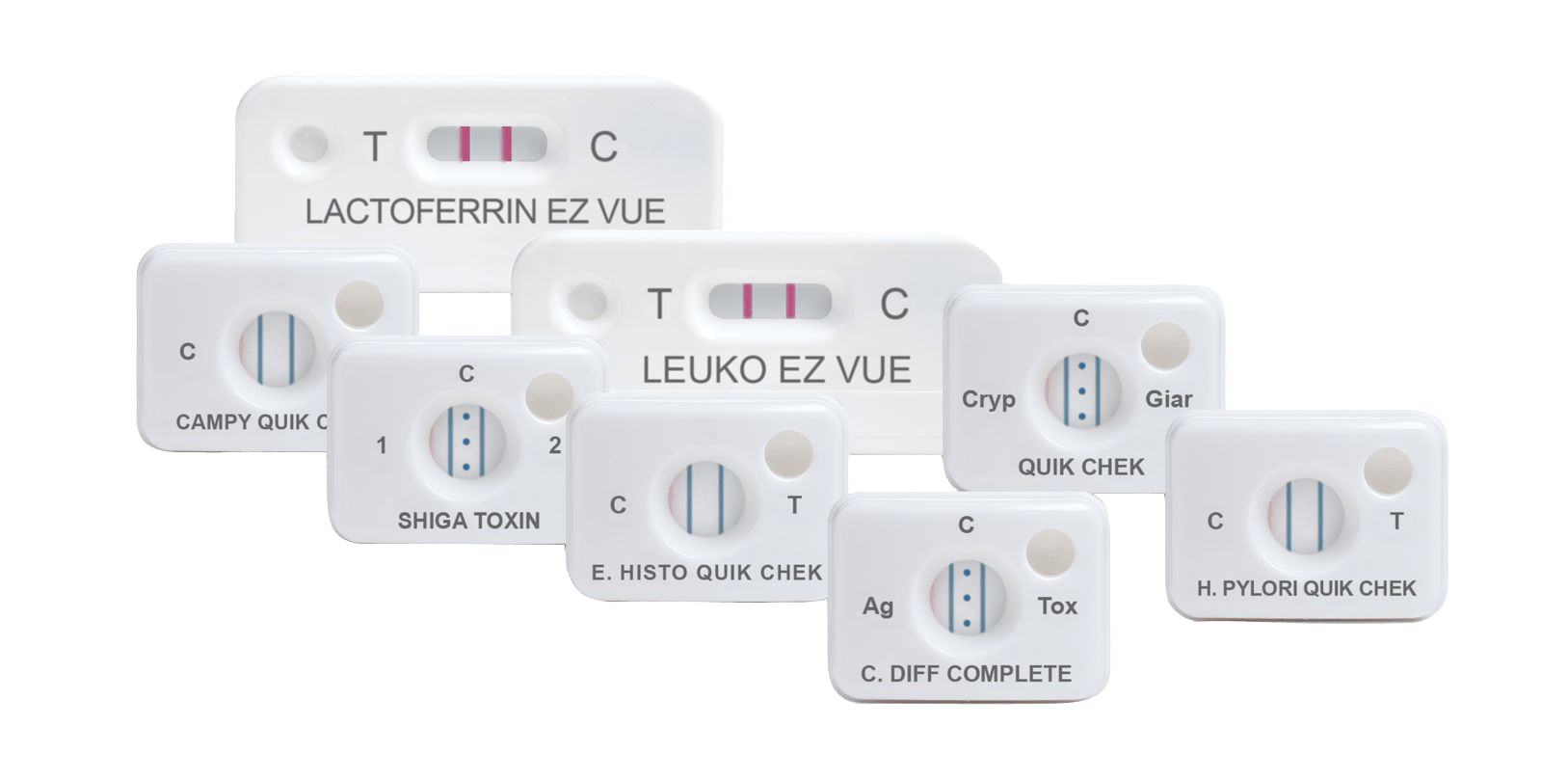
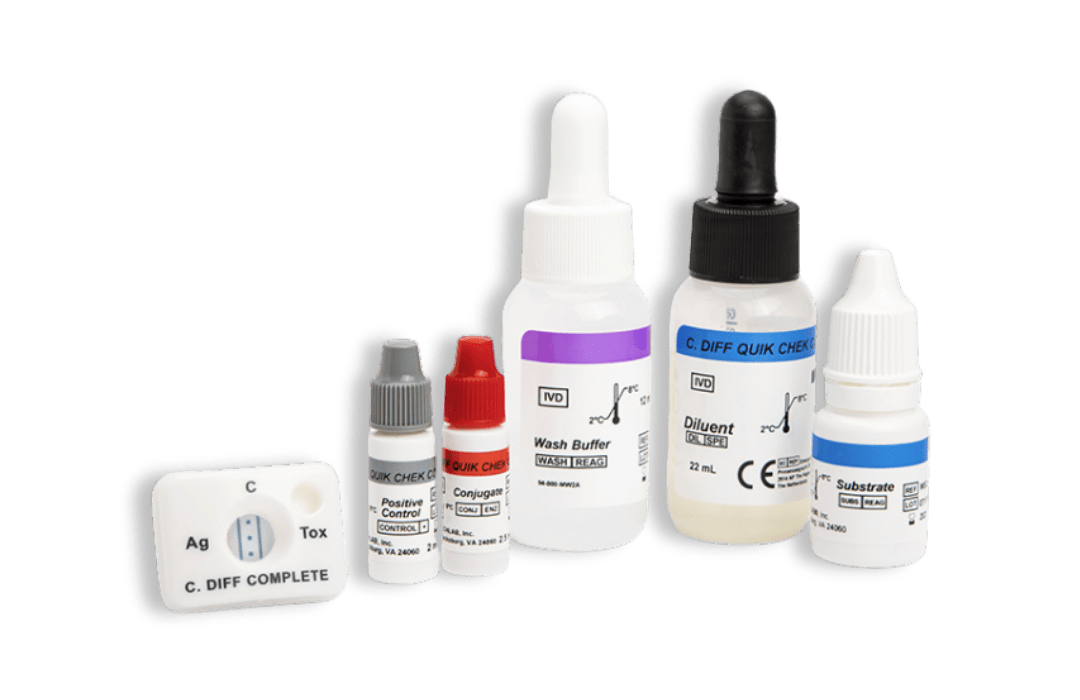
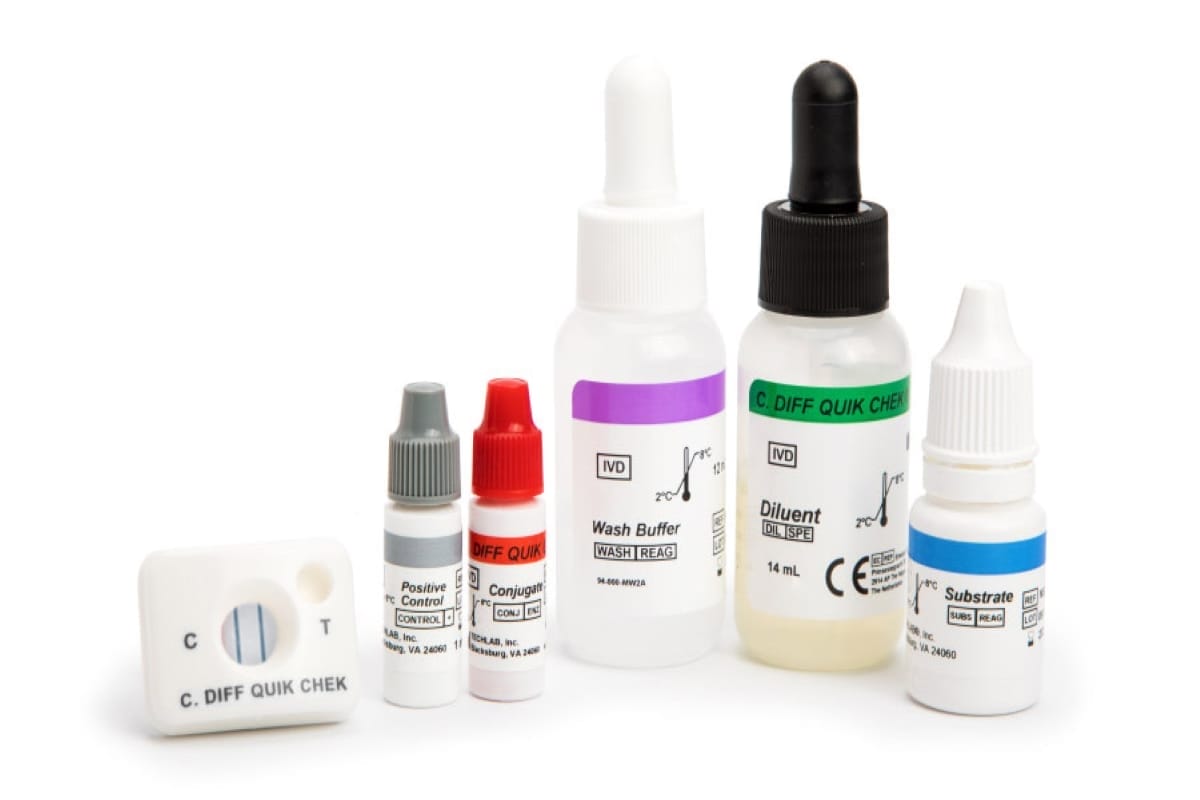
C. DIFF QUIK CHEK ®
30390
- Single test membrane EIA technology in a cassette
- Results in <30 minutes
- Suitable for smaller numbers of samples or for ‘out-of-workflow’ testing
- Sensitivity and Negative Predictive Value (NPV) of GDH are equivalent to toxigenic culture
- A positive result in the test, which is highly specific for the glutamate dehydrogenase of C. difficile, confirms the presence of this organism in a fecal specimen; a negative result indicates the absence of the organism. A positive result should be followed by a toxin-specific test to confirm the presence of toxigenic C. difficile.
*We can automate your ELISA solutions
Una Health have partnered with Aspect Scientific, to deliver the best-in-class ELISA automation for Enterics, Infectious Diseases, Immunology and Biochemistry assays.
Aspect Scientific’s market-leading DYNEX ELISA automation systems are capable of automating almost any ELISA assay. From single microplate readers and washers, up to fully automated 2, 4 & 12 plate systems, Aspect Scientific offer a range of solutions designed to fit your workload, throughput requirements and your budget.
Please get in contact with us at enquiries@unahealth.co.uk for more details on the automation options available.