C. difficile Toxin A/B Testing – TECHLAB®
The gold standard in enteric pathogen detection
Compatible with ECCMID guidelines
Clostridioides difficile (C. difficile) is the major cause of nosocomial diarrhoea and pseudomembranous colitis. The bacterium produces spores and infection usually occurs when antibiotics alter or kill the normal gut microflora, allowing the germination of spores into vegetative cells. Once C. difficile growth begins, toxins A and B may be produced, causing diarrhoea and colitis.
Product Overview
Toxin A/B tests from TECHLAB help detect toxin A, an enterotoxin and toxin B, a cytotoxin, which may be produced once C. difficile growth begins, causing the symptoms of the infection.
Current UK guidelines advise that organisations adhere to a two-stage testing approach which consists of a test to screen for glutamate dehydrogenase (GDH) using EIA, NAAT, or PCR, followed by a toxin test. What is important to note is that algorithm testing provides a more complete diagnostic picture than molecular testing alone. Molecular assays only indicate if a gene is present, not if disease-causing toxins are being produced, hence the importance of following best practice in C. difficile testing with a Toxin A/B test.
The Toxin A/B test options from TECHLAB are:
C. DIFFICILE TOX A/B II™
T5015 / T5015B
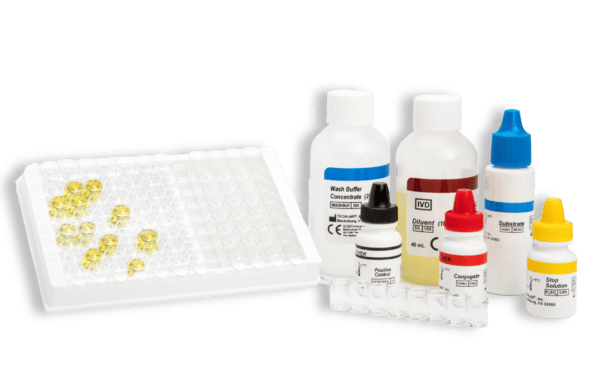
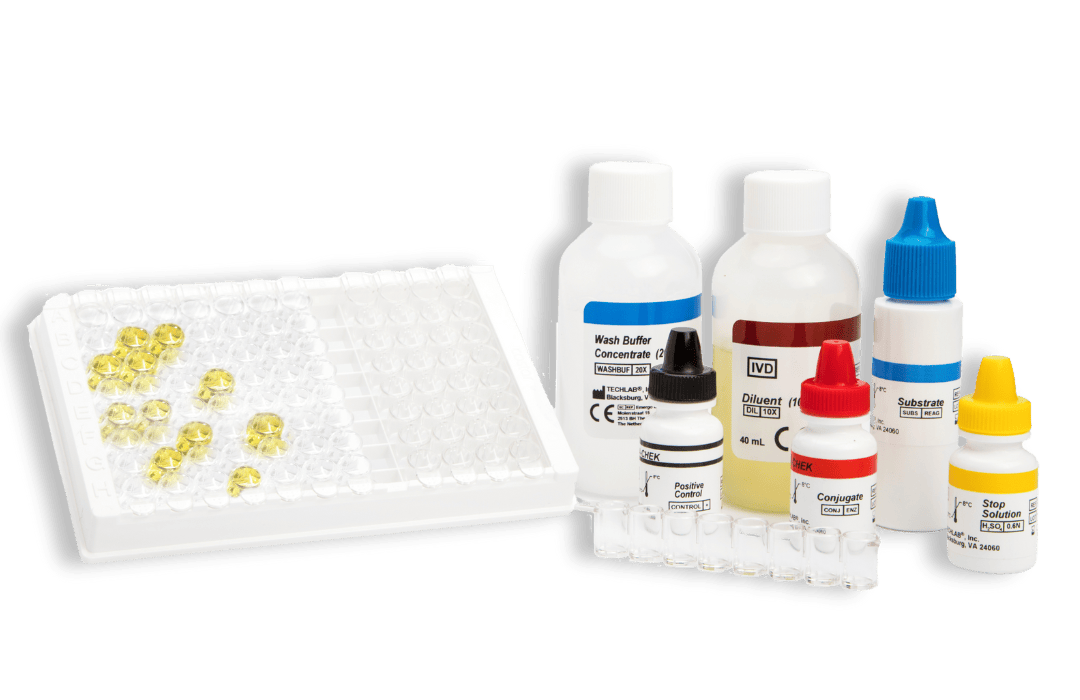
- Gold standard ELISA assay for the detection of Clostridium difficile toxin A and toxin B in faecal specimens
- Utilises highly specific antibodies
- High correlation with tissue culture, but much faster
- Sample type: Fresh and frozen faecal sample (unpreserved)
- Time to result: 1 hour
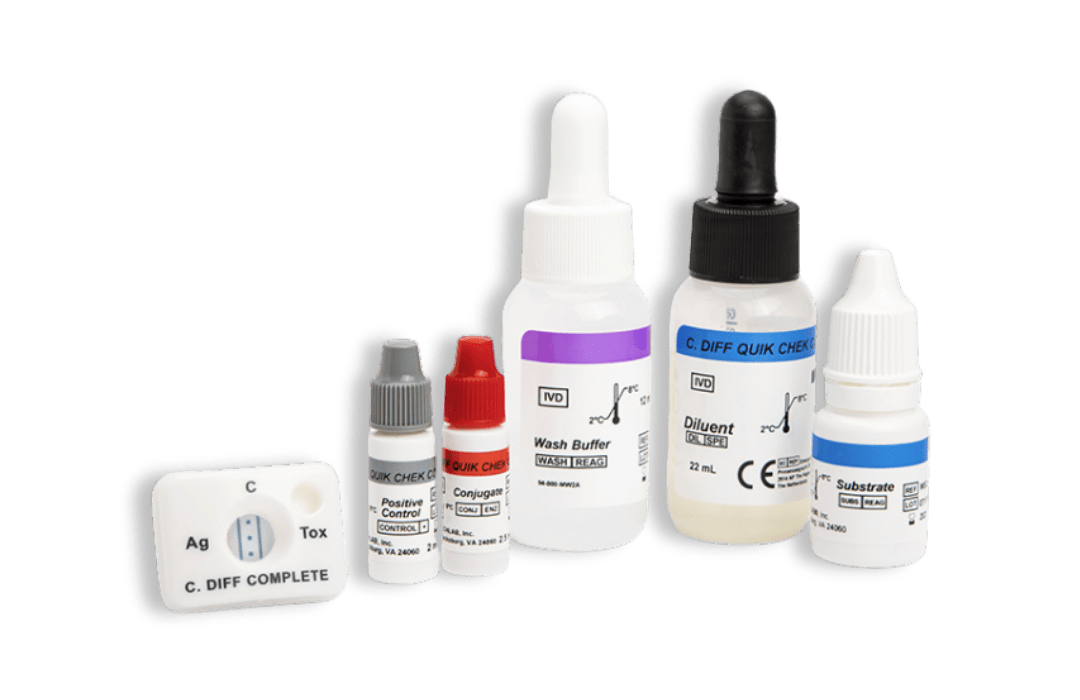
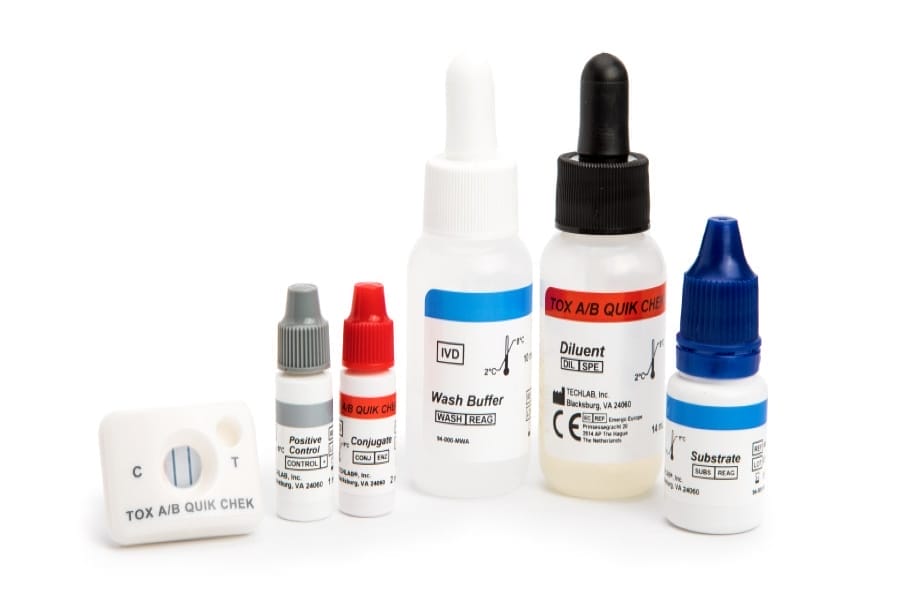
TOX A/B QUIK CHEK®
30394
- Rapid enzyme immunoassay that uses antibodies specific for toxins A and B of C. difficile.
- easy-to-read results
- Sample type: Fresh and frozen faecal sample (unpreserved), Cary Blair, C&S
- Time to result: <30 minutes
C. DIFFICILE TOX-B TEST
T5003
- Tissue culture assay for the detection of Clostridium difficile toxin B in faecal specimens
- Detects the presence of cytotoxic activity (cell rounding) in fecal specimens and confirms the identification of C. difficile toxin by using specific antitoxin. Reagents in the kit include toxin control, specific antitoxin, Phosphate Buffered Saline, and diluent.
- Minimises repeat testing of specimens containing high toxin levels